Galvanic Cells | Chemistry Optional Notes for UPSC PDF Download
Chemistry of Batteries
- Chemistry is the driving force behind the magic of batteries. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic cell consists of at least two half cells, a reduction cell and an oxidation cell. Chemical reactions in the two half cells provide the energy for the galvanic cell operations.
- Each half cell consists of an electrode and an electrolyte solution. Usually the solution contains ions derived from the electrode by oxidation or reduction reaction.
- A galvanic cell is also called a voltaic cell. The spontaneous reactions in it provide the electric energy or current.
- Two half cells can be put together to form an electrolytic cell, which is used for electrolysis. In this case, electric energy is used to force nonspontaneous chemical reactions.
Oxidation-Reduction Reactions
- Many definitions can be given to oxidation and reduction reactions. In terms of electrochemistry, the following definition is most appropriate, because it let's us see how the electrons perform their roles in the chemistry of batteries.
Note
Loss of electrons is oxidation (LEO), and gain of electrons is reduction (GER).
- Oxidation and reduction reactions cannot be carried out separately. They have to appear together in a chemical reaction. Thus oxidation and reduction reactions are often called redox reactions. In terms of redox reactions, a reducing agent and an oxidizing agent form a redox couple as they undergo the reaction:
Oxidant + ne− → Reductant
Reducant → Oxidant + ne− - An oxidant is an oxidizing reagent, and a reductant is a reducing agent. The
reductant | oxidant or oxidant | reductant
Two members of the couple are the same element or compound, but of different oxidation state.
Copper-Zinc Voltaic Cells
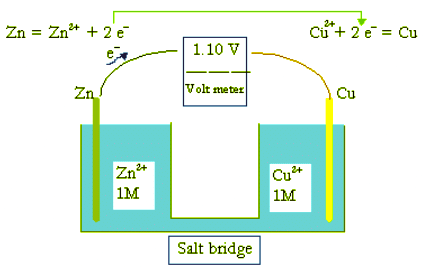
- As an introduction to electrochemistry let us take a look at a simple voltaic cell or a galvanic cell. When a stick of zinc (Zn) is inserted in a salt solution, there is a tendency for Zn to lose electrons according to the reaction,
Zn → Zn2+ + 2e− - The arrangement of a Zn electrode in a solution containing Zn2+ ions is a half cell, which is usually represented by the notation:
Zn|Zn2+, - Zinc metal and Zn2+ ion form a redox couple, Zn2+ being the oxidant, and Zn the reductant. The same notation was used to designate a redox couple earlier. Similarly, when a stick of copper (Cu) is inserted in a copper salt solution, there is also a tendency for Cu to lose electrons according to the reaction,
Cu → Cu2+ + 2e−
This is another half cell or redox couple: Cu|Cu2+ - However, the tendency for Zn to lose electrons is stronger than that for copper. When the two cells are connected by a salt bridge and an electric conductor as shown to form a closed circuit for electrons and ions to flow, copper ions (Cu2+) actually gain electrons to become copper metal. The reaction and the redox couple are respectively represented below,
Cu2+ + 2e− → Cu, Cu2+|Cu - This arrangement is called a galvanic cell or battery as shown here. In a text form, this battery is represented by,
Zn|Zn2+||Cu2+|Cu,
in which the two vertical lines ( || ) represent a salt bridge, and a single vertical line ( | ) represents the boundary between the two phases (metal and solution). Electrons flow through the electric conductors connecting the electrodes, and ions flow through the salt bridge. When
[Zn2+] = [Cu2+] = 1.0 M,
the voltage between the two terminals has been measured to be 1.100 V for this battery. - A battery is a package of one or more galvanic cells used for the production and storage of electric energy. The simplest battery consists of two half cells, a reduction half cell and an oxidation half cell.
Oxidation and Reduction Reactions - A Review
- The overall reaction of the galvanic cell is
Zn + Cu2+ → Zn2+ + Cu
Note that this redox reaction does not involve oxygen at all. For a review, note the following:
- Oxidant + ne− → Reductant
- Example: Cu2+ + 2e− → Cu
- Cu2+ is the oxidizing agent and Cu the reducing agent.
- Reductant → Oxidant + ne−
- Example: Zn → Zn2+ + 2e− .
- Zn is the reducing agent, and Zn2+ the oxidizing agent.
Theoretically, any redox couple may form a half-cell, and any two half cells may combine to give a battery, but we have considerable technical difficulty in making some couples into a half cell.
FAQs on Galvanic Cells - Chemistry Optional Notes for UPSC
| 1. What is the role of oxidation-reduction reactions in batteries? |  |
| 2. How do galvanic cells work in batteries? |  |
| 3. What are some examples of oxidation and reduction reactions in batteries? |  |
| 4. How do oxidation and reduction reactions affect the battery's lifespan? |  |
| 5. What is the significance of understanding oxidation-reduction reactions in batteries? |  |




















