Best Study Material for UPSC Exam
UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Nernst Equation
Nernst Equation | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Introduction |

|
| Electric Work and Gibb's Free Energy |

|
| The General Nernst Equation |

|
| Solved Examples |

|
Introduction
- Electrochemistry deals with cell potential as well as energy of chemical reactions. The energy of a chemical system drives the charges to move, and the driving force gives rise to the cell potential of a system called galvanic cell. The energy aspect is also related to the chemical equilibrium. All these relationships are tied together in the concept of the Nernst equation.
- Walther H. Nernst (1864-1941) received the Nobel prize in 1920 "in recognition of his work in thermochemistry". His contribution to chemical thermodynamics led to the well known equation correlating chemical energy and the electric potential of a galvanic cell or battery.
Question for Nernst Equation
Try yourself:
What is the Nernst equation used to calculate?View Solution
Electric Work and Gibb's Free Energy
- Energy takes many forms: mechanical work (potential and kinetic energy), heat, radiation (photons), chemical energy, nuclear energy (mass), and electric energy. A summary is given regarding the evaluation of electric energy, as this is related to electrochemistry.
Electric Work
- Energy drives all changes including chemical reactions. In a redox reaction, the energy released in a reaction due to movement of charged particles gives rise to a potential difference. The maximum potential difference is called the electromotive force (EMF), E, and the maximum electric work W is the product of charge q in Coulomb (C), and the potential DE in Volt (= J / C) or EMF.
- WJ = qDECJ/C (units)
- Note that the EMF DE is determined by the nature of the reactants and electrolytes, not by the size of the cell or amounts of material in it. The amount of reactants is proportional to the charge and available energy of the galvanic cell.
Gibb's Free Energy
- The Gibb's free energy DG is the negative value of maximum electric work,
ΔG = −W
= −q Δ E (1), (2) - A redox reaction equation represents definite amounts of reactants in the formation of also definite amounts of products. The number (n) of electrons in such a reaction equation is related to the amount of charge transferred when the reaction is completed. Since each mole of electron has a charge of 96485 C (known as the Faraday's constant, F),
q = nF
and,
ΔG = −nF Δ E - At standard conditions,
ΔG∘ = −nF ΔE∘
The General Nernst Equation
- The general Nernst equation correlates the Gibb's Free Energy DG and the EMF of a chemical system known as the galvanic cell. For the reaction
aA + bB ⇌ cC + dD
and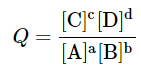
- It has been shown that
DG = DG∘ + RT ln Q
and
DG = − nF DE
Therefore
−nF DE = −nF DE∘ + RT ln Q - where R, T, Q and F are the gas constant (8.314 J mol-1 K-1), temperature (in K), reaction quotient, and Faraday constant (96485 C) respectively. Thus, we have
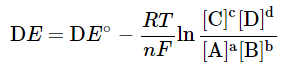
- This is known as the Nernst equation. The equation allows us to calculate the cell potential of any galvanic cell for any concentrations. Some examples are given in the next section to illustrate its application. It is interesting to note the relationship between equilibrium and the Gibb's free energy at this point. When a system is at equilibrium, DE = 0, and Qeq = K. Therefore, we have,
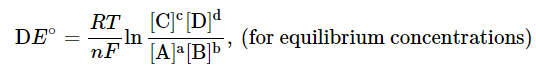
- Thus, the equilibrium constant and DE° are related.
The Nernst Equation at 298 K
- At any specific temperature, the Nernst equation derived above can be reduced into a simple form. For example, at the standard condition of 298 K (25°), the Nernst equation becomes
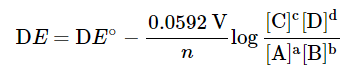
- Please note that log is the logarithm function based 10, and ln, the natural logarithm function. For the cell
Zn|Zn2+||H+ |H2|Pt - we have a net chemical reaction of
Zn(s) + 2H+ → Zn2+ + H2(g)
and the standard cell potential ΔE° = 0.763. - If the concentrations of the ions are not 1.0 M, and the H2 pressure is not 1.0 atm, then the cell potential DE may be calculated using the Nernst equation:
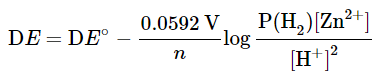
with n = 2 in this case, because the reaction involves 2 electrons. The numerical value is 0.0592 only when T = 298 K. This constant is temperature dependent. Note that the reactivity of the solid Zn is taken as 1. If the H2 pressure is 1 atm, the term P(H2) may also be omitted. The expression for the argument of the log function follows the same rules as those for the expression of equilibrium constants and reaction quotients. - Indeed, the argument for the log function is the expression for the equilibrium constant K, or reaction quotient Q. When a cell is at equilibrium, ΔE = 0.00 and the expression becomes an equilibrium constant K, which bears the following relationship:
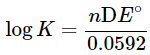
where ΔE° is the difference of standard potentials of the half cells involved. A battery containing any voltage is not at equilibrium. - The Nernst equation also indicates that you can build a battery simply by using the same material for both cells, but by using different concentrations. Cells of this type are called concentration cells.
 |
Download the notes
Nernst Equation
|
Download as PDF |
Download as PDF
Solved Examples
Example 1: Calculate the EMF of the cell
Zn(s)| Zn2 + (0.024M) || Zn2+(2.4M) | Zn(s)
Ans:
Using the Nernst equation: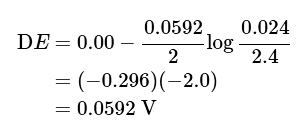 (3), (4), (5)
(3), (4), (5)
Discussion
- Understandably, the Zn2+ ions try to move from the concentrated half cell to a dilute solution. That driving force gives rise to 0.0592 V. From here, you can also calculate the energy of dilution.
If you write the equation in the reverse direction,
Zn2+ (0.024M) → Zn2+ (2.4M), - its voltage will be -0.0592 V. At equilibrium concentrations in the two half cells will have to be equal, in which case the voltage will be zero.
Example 2: Show that the voltage of an electric cell is unaffected by multiplying the reaction equation by a positive number.
Ans: Assume that you have the cell
Mg | Mg2+ || Ag+ | Ag
and the reaction is:
Mg+2 Ag+ → Mg2+ + 2Ag
Using the Nernst equation
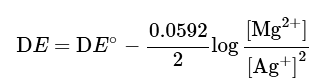
If you multiply the equation of reaction by 2, you will have
2Mg + 4Ag+ → 2Mg2+ + 4Ag
Note that there are 4 electrons involved in this equation, and n = 4 in the Nernst equation: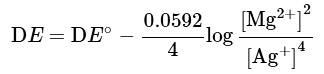
which can be simplified as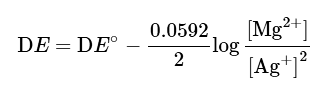
Thus, the cell potential DE is not affected.
The document Nernst Equation | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Nernst Equation - Chemistry Optional Notes for UPSC
| 1. What is the significance of the General Nernst Equation in the study of electric work and Gibbs free energy? |  |
| 2. How is the Nernst Equation applied in practical situations? |  |
Ans. The Nernst Equation finds practical applications in various fields. In electrochemistry, it is used to determine the equilibrium potential of a cell, which helps in predicting the direction of chemical reactions. It is also utilized in the design and optimization of electrochemical devices such as batteries, fuel cells, and sensors. Furthermore, the Nernst Equation is employed in pharmaceutical research to study drug-receptor interactions and in environmental science to measure the concentration of specific ions in water samples.
| 3. Can you explain the relationship between the Nernst Equation and Gibbs free energy? |  |
Ans. The Nernst Equation and Gibbs free energy are closely related. The Nernst Equation helps in calculating the equilibrium potential of a cell, which is based on the concentrations of the reactants and products involved. This potential, in turn, influences the value of Gibbs free energy for the electrochemical reaction. By comparing the calculated cell potential with the standard cell potential, it is possible to determine whether the reaction is spontaneous or non-spontaneous and to calculate the change in Gibbs free energy.
| 4. How does the complexity of the Nernst Equation impact its practical application? |  |
Ans. The complexity of the Nernst Equation lies in its dependence on various factors such as the temperature, Faraday's constant, and the gas constant. These factors need to be accurately accounted for in order to obtain precise results. Additionally, the Nernst Equation assumes ideal conditions, which may not always be the case in practical situations. Therefore, while the Nernst Equation provides valuable insights, its application requires careful consideration of all the variables and limitations.
| 5. What are the limitations of the Nernst Equation? |  |
Ans. The Nernst Equation has certain limitations that need to be taken into account. It assumes that the reaction is taking place at standard conditions and that the ions involved are in dilute solution. It does not consider factors such as the presence of complex ions, changes in activity coefficients, or non-ideality of the system. Additionally, the Nernst Equation is applicable only to reversible reactions and may not accurately predict the behavior of irreversible reactions. Therefore, while it is a useful tool, it should be used cautiously and in conjunction with other relevant equations and considerations.
Related Searches






















