UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > M-CPBA (meta-chloroperoxybenzoic acid)
M-CPBA (meta-chloroperoxybenzoic acid) | Chemistry Optional Notes for UPSC PDF Download
m-Chloroperoxybenzoic Acid (m-CPBA) For The Epoxidation of Alkenes
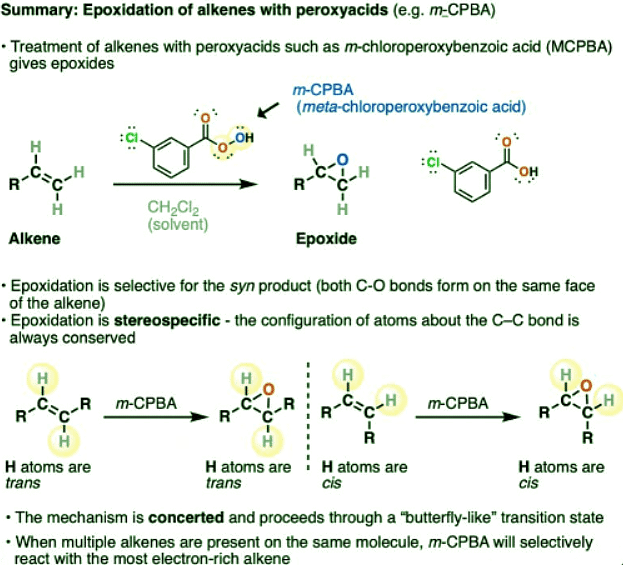
- m-CPBA (meta-chloroperoxybenzoic acid) is a useful reagent for the formation of epoxides from alkenes (note – often just called, m-chloroperbenzoic acid, without the “oxy”)
- In this reaction, the C-C pi bond is broken, and two new C-O single bonds are formed on the same face of the alkene pi-bond. Since both bonds are formed on the same face, this is an example of a syn addition.
- The weak O-O bond in m-CPBA is also broken. The -OH of the peroxyacid is the source of the oxygen in the new epoxide
- Epoxidation of alkenes with m-CPBA is an example of a stereospecific reaction. The configuration of atoms about the C–C bond is always conserved. The reaction proceeds through a concerted transition state.
- Other peroxyacids such as peracetic acid, perbenzoic acid, and trifluoroperacetic acid are also effective reagents for epoxidation of alkenes.
- Alkynes do not undergo reaction with m-CPBA to give epoxides
- m-CPBA is also useful for the Baeyer-Villiger oxidation, a reaction that converts ketones to esters.
Epoxidation of Alkenes With Peroxyacids
- When alkenes are treated with peroxyacids such as meta-chloroperoxybenzoic acid (m-CPBA), two new C-O bonds are formed and a C-C pi bond is broken, resulting in the formation of an epoxide. (An O–O bond from m-CPBA is also broken, which is the source of the oxygen in the epoxide. The byproduct is m-chlorobenzoic acid).
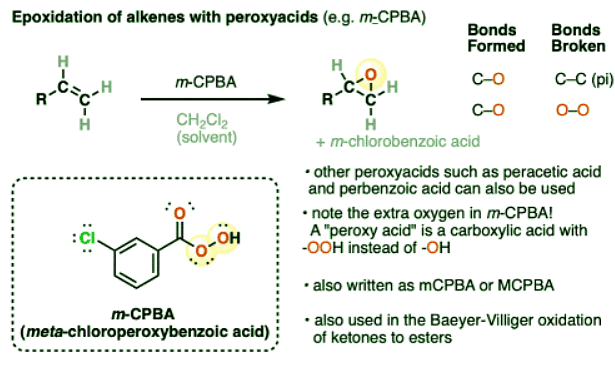
- Epoxides, also known as oxiranes, are 3-membered cyclic ethers. Since the inner bond angles of epoxides (approximately 60°) deviate significantly from the preferred tetrahedral geometry around carbon (109.5°) , they possess considerable ring strain (about 13 kcal/mol) and undergo a large number of useful ring-opening reactions with nucleophiles.
- Epoxidation of alkenes with peroxyacids such as m-CPBA always occurs in such a way that both C-O bonds are formed on the same face of the alkene, an outcome known as “syn addition”.
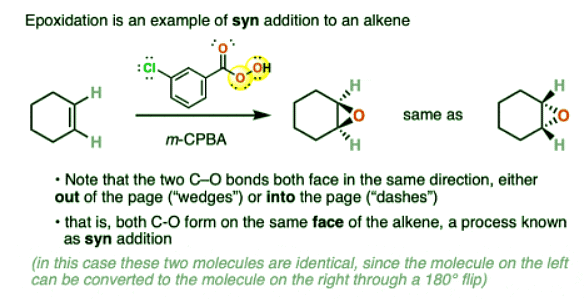
- You can tell that addition is syn here because the new C-O bonds are both drawn as “wedges” (pointing out of the page) or “dashes” (pointing into the page).
- In many other cases this will result in the formation of a pair of enantiomers or diasteromers, depending on the structure of the starting alkene.
- Epoxidation of alkenes with peroxyacids never results in anti addition.
- It is incorrect to draw the product of this epoxidation reaction as having one “wedge” C-O and one “dash” C-O, since this would represent anti addition.
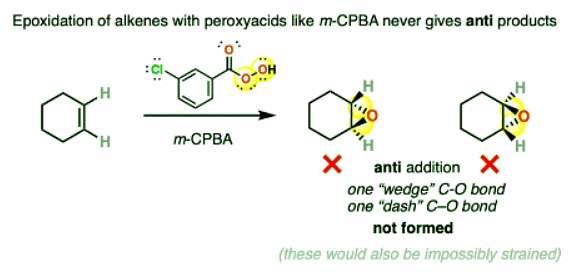
- (BTW if you try to make a model, you will see that this outcome would also be impossibly strained)
Question for M-CPBA (meta-chloroperoxybenzoic acid)Try yourself: Which of the following statements is true regarding the epoxidation of alkenes with m-CPBA?View Solution
Epoxidation of Alkenes Is Stereospecific
- In epoxidation reactions, the configuration of atoms about the alkene is always conserved.
- For example, the trans alkene below gives only the trans product (as an equal mixture of enantiomers). Note that in product A, the trans arrangement of C–H bonds about the C–C bond has been conserved.
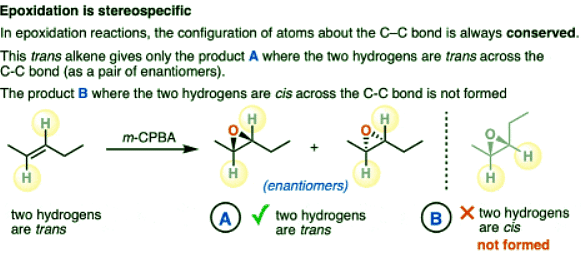
- The product B where the two C-H bonds are cis to the C-C bond is not formed. (Note that this product B would be the diastereomer of product A).
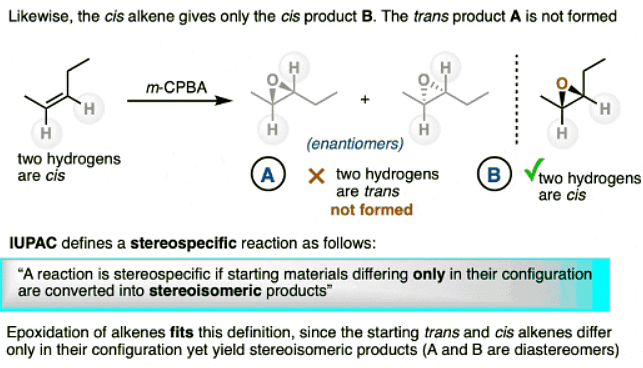
- Likewise, the cis alkene gives only product B. (Product A, diastereomers of product B, are not formed.)
The Mechanism of Epoxidation of Alkenes With m-CPBA
- Now that we’ve identified the bonds that form and break, and the expected stereochemistry for this reaction, we can start to ask ourselves how this reaction actually works.
- First of all: which oxygen from m-CPBA is transferred to give the epoxide?
- Isotopic labelling studies can verify that the oxygen of the epoxide comes from the OH of the peroxyacid, not the interior O connected to the C=O.
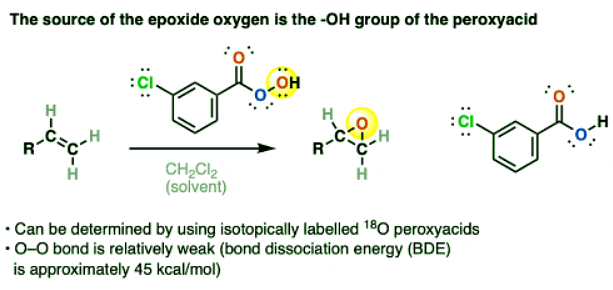
- One driving force for the reaction is breakage of the relatively weak O–O and C-C pi bonds (bond dissocation energies of about 45 kcal/mol and 60 kcal/mol, respectively) in exchange for two relatively strong C-O sigma bonds (about 90 kcal/mol).
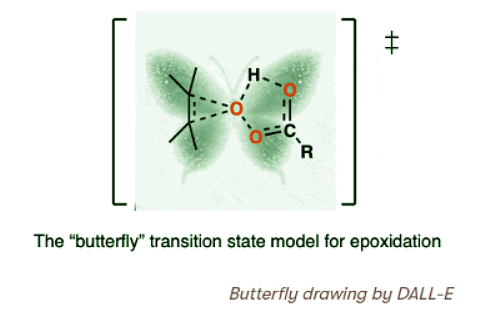
- The transition state that has been proposed for the reaction of m-CPBA (and other peroxyacids) with alkenes has become known as the “butterfly mechanism”, owing to the four(!) partial bonds around the central oxygen that give the appearance of a large-winged insect.
- This reaction gets my vote for the busiest one-step arrow-pushing mechanism in all of introductory organic chemistry:
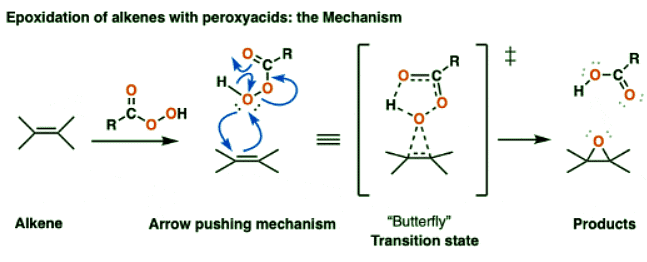
- Let’s break down everything that happens here in this one (!) step:
- The C–C pi bond breaks
- Two new C–O single bonds form
- The (weak) O–OH bond breaks
- Meanwhile, the resulting carboxylic acid is transposed: a new C–O pi bond forms, while the existing C-O pi bond acts as a base to remove a proton from the terminal oxygen.
- This “butterfly” mechanism accounts for the following experimental observations:
- The reaction is first-order in both peroxyacid and alkene (second order overall)
- The rate of reaction is not sensitive to the polarity of the solvent (making a charged carbocation intermediate unlikely)
- It also accounts for the stereospecificity of the reaction. We would not expect a reaction that proceeds through a carbocation intermediate to be stereospecific, for example. (The reaction of HCl with alkenes is not stereospecific, for example)
- Two additional facts are worth noting.
- Electron-rich alkenes react more quickly than electron poor alkenes, and
- adding electron-withdrawing groups to the R group of the peroxyacid make it more reactive. (The added chlorine on the 3-position of the benzene ring makes it more electron-withdrawing and therefore more reactive, relative to plain-ol’ peroxybenzoic acid).
Question for M-CPBA (meta-chloroperoxybenzoic acid)Try yourself: Which alkene will undergo epoxidation first when treated with m-CPBA?View Solution
Which Alkene Will React?
- Let’s imagine we have a molecule containing multiple C-C pi bonds that is treated with m-CPBA.
- Is it possible to get epoxidation to happen at just one alkene pi-bond, or do we just end up with a mixture of products?
- What is observed is that the most electron-rich alkene undergoes epoxidation first, with remarkably good selectivity.
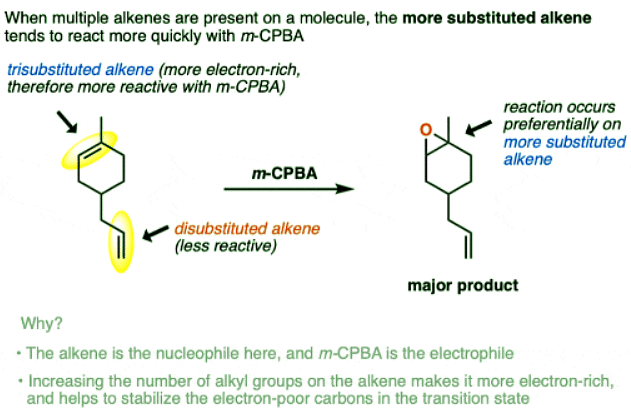
- “Most electron-rich” generally means the alkene which has the most carbon atoms directly attached to the alkene (i.e. “most substituted”) so long as they are not electron-withdrawing groups.
- For instance, in the alkene above, it’s the trisubstituted alkene preferentially undergoes epoxidation while the mono-substituted alkene is untouched.
- This might seem counter-intuitive since we might expect that a more substituted alkene is more sterically hindered, but in practice the rate of reaction is more sensitive to electronic effects (i.e. electron-rich vs. electron-poor) than steric effects.
Stereoselectivity
- When epoxidation is performed on an alkene containing pre-existing chiral centers, then there is the potential for the formation of stereoisomers. That’s because the two faces of the alkene will not be equivalent steric environments, as seen from the perspective of electrophile (such as m-CPBA).
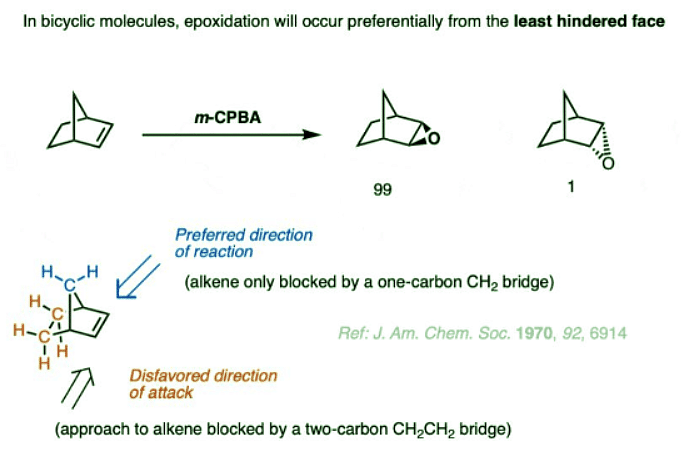
- A particularly striking example is the bicyclic alkene below. When it is treated with m-CPBA, a 99:1 mixture of products (diastereomers) is formed. The reason for the high selectivity is that the electrophile (m-CPBA) only encounters a single CH2 group in its approach to the top face (favored), whereas it encounters a two-carbon bridge in is approach on the bottom face (disfavored).
- In this case, the result is a 99:1 ratio of products favoring attack on the least hindered face of the alkene.
- There is a more systematic method for naming faces of alkenes, (known as Re and Si ) which we will not get into at present.
Other Epoxidation Reagents
- Other peroxyacids have been used for epoxidation reactions, such as peroxyacetic acid, peroxybenzoic acid, and trifluoroperoxyacetic acid.
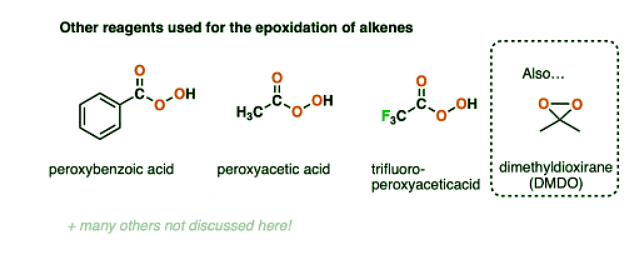
- Just as making the alkene more electron-rich increases the rate of reaction, it has also been observed that adding electron-withdrawing reagents to the electrophile (peroxyacid) increases the rate of reaction.
- The epoxidation of alkenes with peroxyacids has been known since 1909, but it was only in the 1960s that m-CPBA started gaining widespread use, which greatly improved the scope of epoxidation reactions.
- Furthermore, although they aren’t often covered in introductory courses, there are other ways of epoxidizing alkenes that don’t involve peroxyacids. Examples include metals such as vanadium, titanium, manganese and others in the presence of peroxides (or hydroperoxides), or the use of reagents such as dimethyldioxirane (DMDO).
Sharpless Asymmetric Epoxidation
- Enantioselective epoxidation of alkenes is also a known process.
- In an enantioselective reaction, one starts with an achiral molecule and adds a chiral reagent that selectively attacks one face of the starting material over the other, resulting in a mixture of products which is enriched in on enantiomer over another.
- One prominent method for the enantioselective epoxidation of alkenes was developed by K. Barry Sharpless (Scripps) and has become known as the Sharpless epoxidation.
- The Sharpless epoxidation involves treating an allylic alcohol with a witches’ brew of titanium isopropoxide [Ti(Oi-Pr)4)] t-butylhydroperoxide (the oxidant) and, depending on which enantiomer is desired, either (S,S) or (R, R) diethyl tartrate (or di-isopropyl tartrate, in some cases).
Here is a simple example.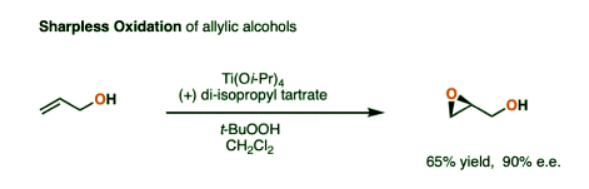
- The reaction is not enantioselective without the OH group on the carbon adjacent to the alkene. (This class of molecules is known as “allylic alcohols”). The hydroxyl group is required to coordinate to the titanium and direct the epoxidation.
Question for M-CPBA (meta-chloroperoxybenzoic acid)Try yourself: Which reagent is commonly used for the epoxidation of alkenes?View Solution
The document M-CPBA (meta-chloroperoxybenzoic acid) | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on M-CPBA (meta-chloroperoxybenzoic acid) - Chemistry Optional Notes for UPSC
| 1. What is m-Chloroperoxybenzoic Acid (m-CPBA) used for? |  |
Ans. m-Chloroperoxybenzoic Acid (m-CPBA) is commonly used as a reagent for the epoxidation of alkenes. It can introduce an oxygen atom into the double bond of an alkene, forming an epoxide.
| 2. How does the epoxidation of alkenes with peroxyacids work? |  |
Ans. The epoxidation of alkenes with peroxyacids involves the transfer of an oxygen atom from the peroxyacid to the double bond of the alkene. This results in the formation of an epoxide, which contains a three-membered ring with an oxygen atom.
| 3. Is the epoxidation of alkenes stereospecific? |  |
Ans. Yes, the epoxidation of alkenes is stereospecific. This means that the stereochemistry of the starting alkene is preserved in the resulting epoxide. The reaction occurs with syn addition, meaning that the oxygen atom is added to the same face of the double bond as the existing substituents.
| 4. What is the mechanism of epoxidation of alkenes with m-CPBA? |  |
Ans. The mechanism of epoxidation of alkenes with m-CPBA involves the formation of a peroxyacid ester intermediate. The alkene attacks the electrophilic carbonyl carbon of m-CPBA, forming a cyclic intermediate. This intermediate then undergoes rearrangement to form the final epoxide product.
| 5. Which alkene will react with m-CPBA? |  |
Ans. m-CPBA can react with a wide range of alkenes, including both terminal and internal alkenes. It is important to note that the reaction is regioselective, meaning that the oxygen atom is preferentially added to the more substituted carbon of the double bond.
Related Searches





















