UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives | Chemistry Optional Notes for UPSC PDF Download
Introduction
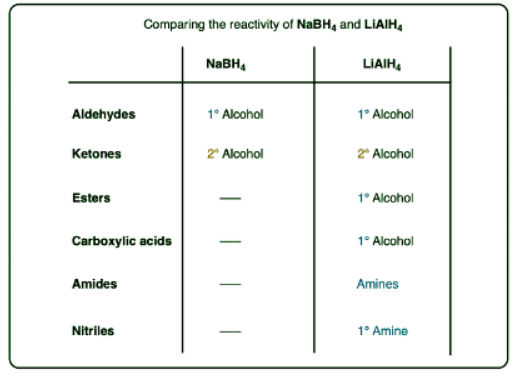
- Lithium aluminum hydride (LiAlH4) is a strong reducing agent similar to, but stronger than, sodium borohydride (NaBH4)
- Like NaBH4, lithium aluminum hydride will reduce aldehydes and ketones to alcohols.
- Unlike NaBH4, it will also reduce carboxylic acids, esters, lactones, acid halides and anhydrides to primary alcohols
- LiAlH4 will also reduce nitriles and amides to amines
- Finally it can open epoxides as well as reduce alkyl halides to alkanes.
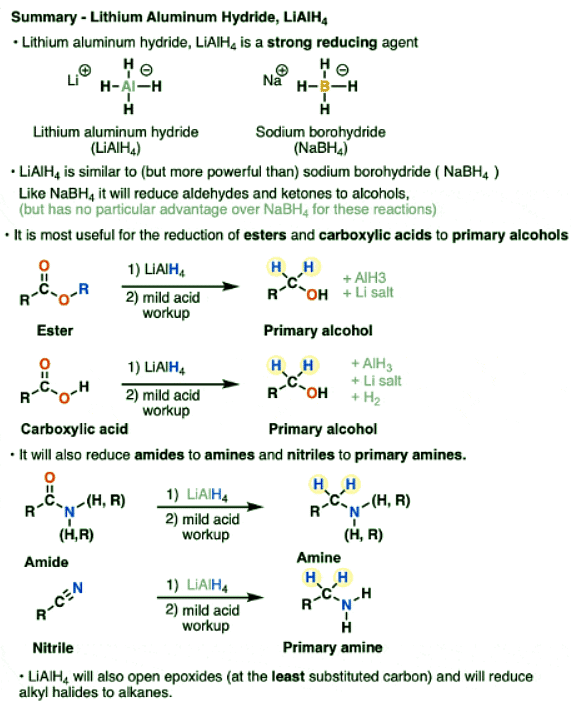
Lithium Aluminum Hydride, LiAlH4
- Lithium aluminum hydride (LiAlH4) is a strong reducing agent with a particular utility for carboxylic acid derivatives.
- If you’re not familiar with what is meant by “reducing agent” in organic chemistry, a refresher can be found here. (See article – Oxidation and Reduction in Organic Chemistry. Short version – a reducing agent forms C-H bonds while breaking C-O bonds).
- Of all reagents, LiAlH4 most resembles sodium borohydride.
- A key property of these reagents is that they act as sources of hydride ion H(-) in their reactions with carbonyls and other electrophiles.
- This might be a good opportunity to remind ourselves that formal charge is not necessarily the same thing as electron density.
- The electronegativity of hydrogen is 2.2.
- The electronegativity of aluminum is 1.61
- So although the aluminum bears a negative formal charge.
- Given that both lithium aluminum hydride and sodium borohydride behave like a source of H(-), see if you can draw a proper electron pushing arrow for the reaction of LiAlH4 with the weak acid, water.
NaBH4 vs LiAlH4
- LiAlH4 will reduce aldehydes and ketones just like NaBH4.
- For practical reasons NaBH4 is much more convenient to use for these reactions and there is no advantage to using LiAlH4 unless you also plan on reducing every other functional group in sight. (As my undergraduate instructor Prof. Walter Szarek was fond of saying, “Using LiAlH4 for this reaction is like using a sledgehammer to kill a fly!”).
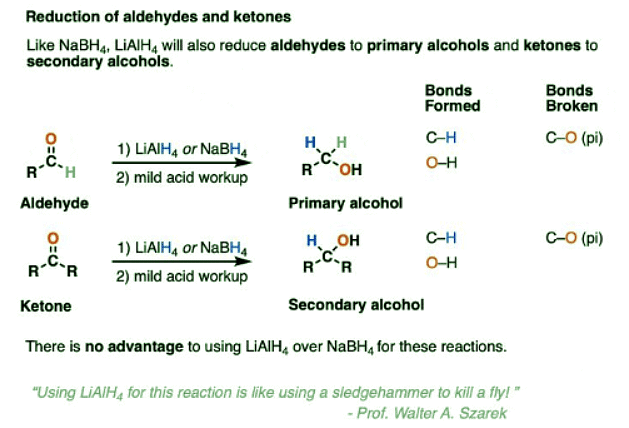
- LiAlH4 will also perform reductions that NaBH4 is unable to do, or at the very least, do them much more quickly.
- One key difference is in the reduction of esters to primary alcohols, which NaBH4 does only slowly (if at all).
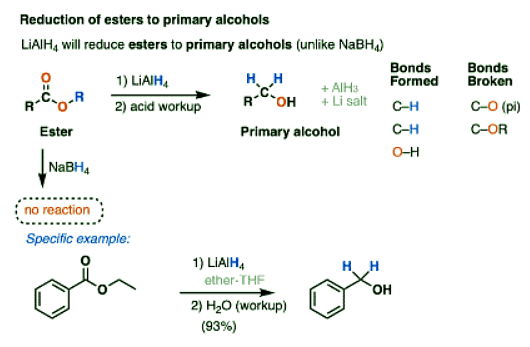
- A detailed, reproducible step-by-step procedure from Organic Syntheses can be found here
- A second reaction that LiAlH4 will perform that NaBH4 will not is the reduction of carboxylic acids to primary alcohols.
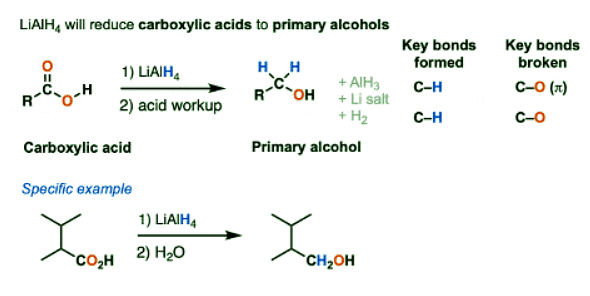
- Other key reactions include:
- acid halides to primary alcohols
- anhydrides to primary alcohols
- As well as reductions of nitriles, amides, epoxides, and alkyl halides
Question for Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid DerivativesTry yourself: Which of the following compounds can be reduced to primary alcohols by lithium aluminum hydride (LiAlH4)?View Solution
Reduction of Carboxylic Acids By LiAlH4 – The Mechanism
- The pKa of H2, the conjugate acid of hydride (H-) is about 36 whereas the pKa of the carboxylic acid is around 4. Since acid-base reactions are favored when a stronger acid will be converted to a weaker acid, this will rapidly generate the carboxylate salt (the conjugate base of the carboxylic acid) and hydrogen gas.
- Acid-base reactions of LiAlH4 tend to be violently exothermic, and generate (flammable) hydrogen gas, besides. For these reasons extreme caution is used when handling LiAlH4 and it is never left out on the bench for any extended period, as it will react with water vapor from the air. Fires can result. [Note 3]. That’s one key reason why NaBH4 is typically used for simple reductions – at cold temperatures, it reacts slowly and controllably with alcoholic solvents, unlike LiAlH4.
- In the presence of most nucleophiles, formation of a carboxylate signals the end of the reaction. We’ve seen that carboxylates will not undergo addition with most nucleophiles. (See article – Nucleophilic Acyl Substitution)
- LiAlH4 is an exception. In the first step, a hydride from aluminum forms a new C-H bond, breaking the C-O pi bond. In the second step, the C-O pi bond is re-formed, resulting in breakage of the C-O sigma bond.
- Nucleophilic acyl substitution on carboxylates is usually extremely difficult due to the strongly basic nature of the O(2-) leaving group, but the strong O-Al bond and aluminum’s strongly Lewis acidic character likely greatly assists here.
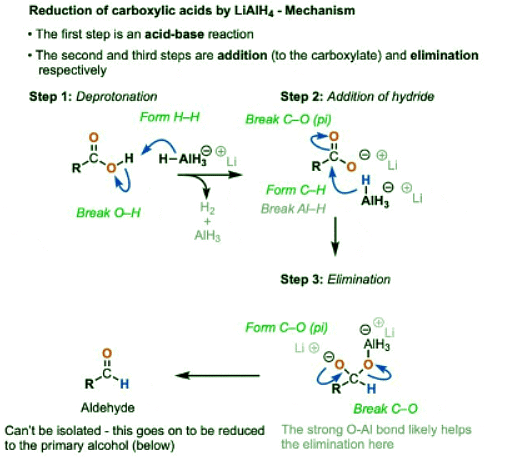
- Elimination results in an aldehyde, which quickly undergoes another reduction. In theory, LiAlH4 has four equivalents of hydride that can be transferred, so it wouldn’t be incorrect to draw AlH3 as the source of hydride here. In practice, an excess of LiAlH4 is generally used, and it’s fine to draw this step of the mechanism as occurring from another equivalent of AlH4(-).
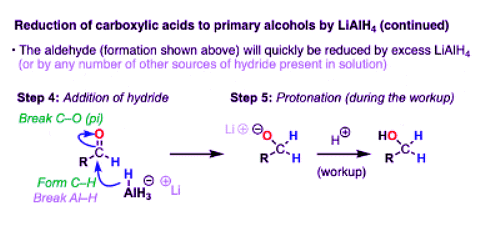
- This gives us the conjugate base of a primary alcohol (an alkoxide) coordinated to aluminum.
- To get our alcohol back, we perform a quench of the reaction with water, which protonates the alkoxide and gives us our neutral alcohol.
- That’s how it works on paper, anyway!
- In practice, the workup is a little bit more complicated because aluminum salts make bitching emulsions that make isolation difficult unless they are completely hydrolyzed (yet another reason to just use NaBH4 if you can!) The Fieser workup is the industry standard, but there are others.
The Mechanism For the Reduction of Esters by LiAlH4
- The mechanism for the reaction of LiAlH4 with esters is even simpler.
- Addition of hydride to the ester [form C-H, break C-O(pi)] followed by elimination of alkoxide [form C-O(pi), break C-O] gives the aldehyde.
- As we’ve seen, LiAlH4 does not stop there. It has enough equivalents of hydride to eat aldehydes for breakfast, lunch, and dinner and have a little bit left over for dessert.
- After the aldehyde has been consumed, a mildly acidic workup gives the primary alcohol.
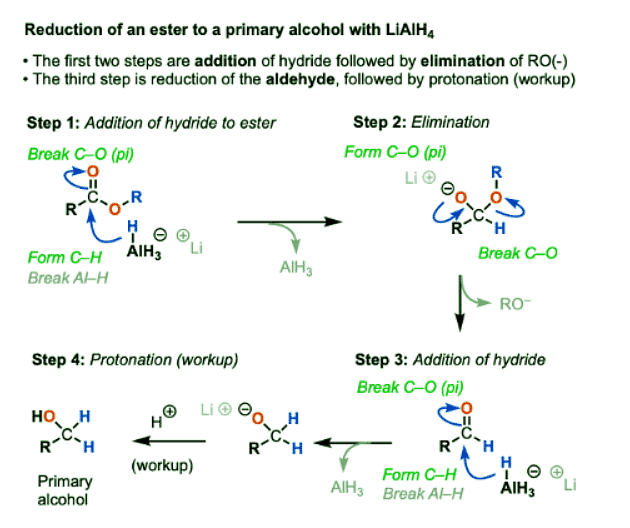
- It is possible to get reduction of esters to stop at the aldehyde stage by adding some groups to the aluminum that serve as a “fat suit”. DIBAL (Di-isobutylaluminum hydride, DIBAL-H) is a classic reagent for these purposes.
- LiAlH4 will also reduce acid halides and anhydrides to primary alcohols through a mechanism similar to that of esters.
- It’s also possible to perform the partial reduction of acid halides to aldehydes through using the related reagent LiAlH(Ot-Bu)3.
Reduction of Amides To Amines by LiAlH4
- Amides are another class of functional groups that are difficult to reduce.
- LiAlH4 will reduce amides to amines, however. NaBH4 won’t touch them.
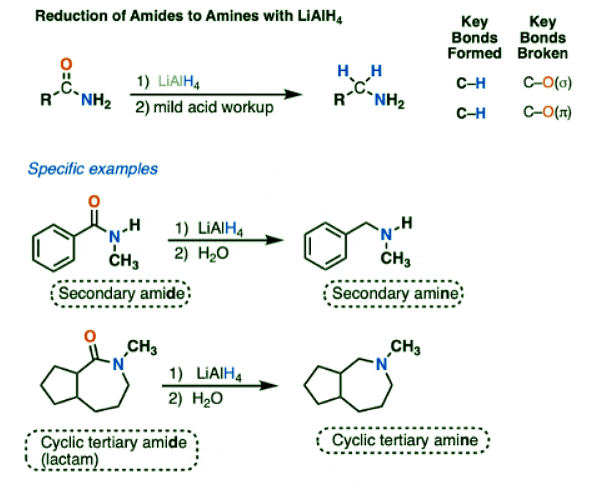
- The N-H bonds of primary and secondary amides are weakly acidic (pKa about 17) and will undergo deprotonation by LiAlH4 to give their conjugate bases followed by reduction to the amine (using the mechanism shown below).
- (Tertiary amides, which lack an acidic hydrogen, will not undergo a preliminary acid-base step.)
- The first step of the reduction of amides is the familiar addition of hydride to carbon (form C-H, break C-O pi) to give a tetrahedral intermediate.
- In nucleophilic acyl substitutions, the second step is elimination of the weakest base to give a new pi bond.
- This usually occurs via formation of C-O (pi) with loss of a leaving group. So might initially guess that the C-O pi bond is the one to form here, eliminating R2N(-) and giving us an intermediate aldehyde.
- But that’s not what happens! Instead, it’s the C-O bond is broken, and a C-N pi bond is formed, giving an iminium intermediate and an alkoxide leaving group.
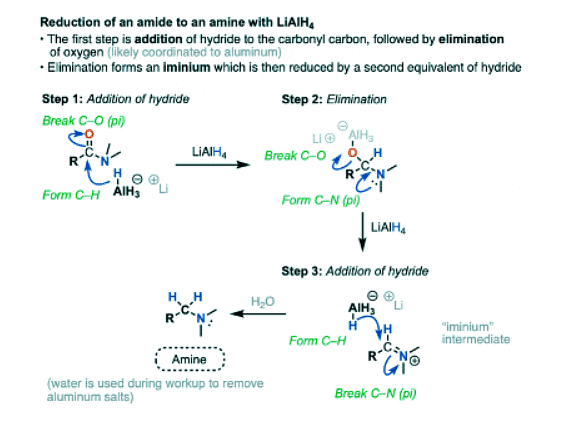
- Like aldehydes, the iminium intermediate doesn’t last long in the presence of all those hydrides.
- The final product obtained after workup (to remove aluminum salts) is an amine.
- The type of amine depends on the amide precursor:
- Primary amides give primary amines
- Secondary amides give secondary amines
- Tertiary amides give tertiary amines
Question for Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid DerivativesTry yourself: Which functional group can LiAlH4 not reduce?View Solution
Reduction of Nitriles to Primary Amines by LiAlH4
- Like the sledgehammer it is, LiAlH4 just keeps smashing through every functional group we’ve thrown in its path. Is there anything it can’t reduce?
- Bring on the next opponent!
- Nitriles require pretty forcing conditions to hydrolyze into carboxylic acids or amides, and their reduction using catalytic hydrogenation requires high temperatures (50-100 °C) and pressures of hydrogen (this ref cites 100 atm of H2).
- Can LiAlH4 do it?
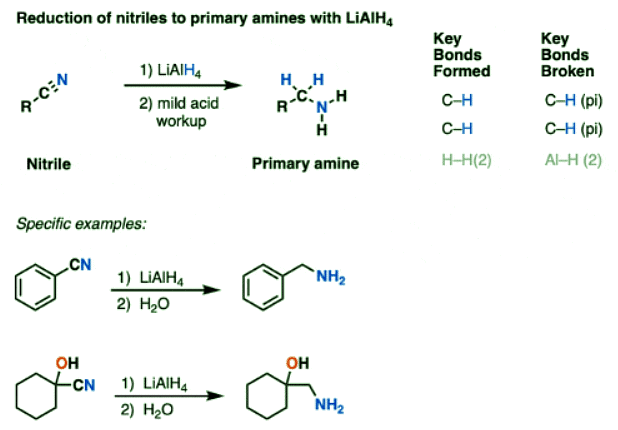
- Of course it can! Nitriles are reduced to primary amines.
- The first step here is another addition, this time to break a C-N (pi) bond and form an imine species. A second addition of hydride gives a negatively charged nitrogen intermediate that is protonated to a primary amine during workup.
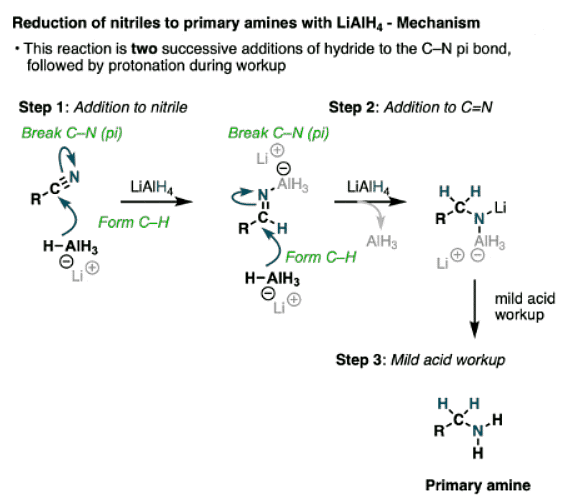
- You may recall that the cyanide ion CN(-) is an excellent nucleophile that will perform SN2 reactions with alkyl halides. So performing an SN2 followed by LiAlH4 reduction is one way of converting alkyl halides into chain-extended primary amines.
Other reactions of LiAlH4
But wait – there’s more!
- LiAlH4 will reduce epoxides to alcohols, which you can think of as being like an SN2 reaction. Like other negatively charged nucleophiles, it adds to epoxides at the least substituted carbon. (See article – Opening of Epoxides With Base)
- LiAlH4 will also, in certain cases, reduce alkyl halides to alkanes, in the order I > Br > Cl > F.
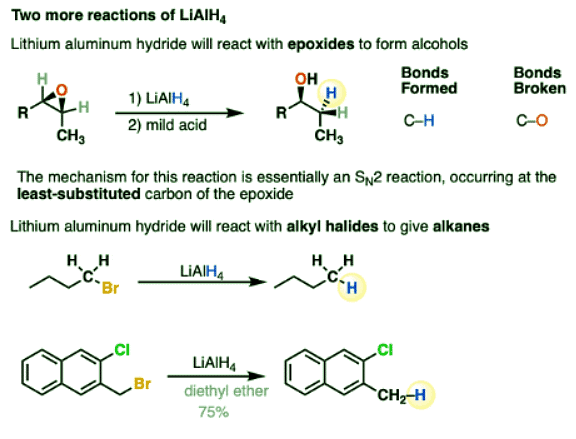
- It can also reduce azides to primary amines.
- One functional group LiAlH4 will not touch is ethers.
Summary
- LiAlH4 is the T-rex of reducing agents. If your functional group absolutely, positively, has to be reduced overnight, use LiAlH4. It’s an all-purpose hydride-adding beast that is powerful, readily available, and relatively cheap.
- If you need a reducing agent that will gently tickle a hydride on to your molecule with a feather-light touch, use something else.
- Various gentler derivatives of LiAlH4 such as DIBAL and LiAlH(Ot-Bu)3 have been developed and are much more selective.
- For reduction of aldehydes and ketones, sodium borohydride (NaBH4) is perfectly fine.
The document Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives - Chemistry Optional Notes for UPSC
| 1. What is the chemical formula of Lithium Aluminum Hydride? |  |
Ans. The chemical formula of Lithium Aluminum Hydride is LiAlH4.
| 2. How does LiAlH4 compare to NaBH4? |  |
Ans. LiAlH4 is a stronger reducing agent compared to NaBH4. It is capable of reducing a wider range of functional groups, including carboxylic acids, esters, amides, and nitriles, whereas NaBH4 is primarily used for reducing aldehydes and ketones.
| 3. What is the mechanism for the reduction of carboxylic acids by LiAlH4? |  |
Ans. The reduction of carboxylic acids by LiAlH4 occurs through a two-step process. First, LiAlH4 reacts with the carboxylic acid to form an intermediate known as an aldehyde. Then, the aldehyde is further reduced by LiAlH4 to give the corresponding primary alcohol.
| 4. How does LiAlH4 reduce esters? |  |
Ans. The reduction of esters by LiAlH4 involves a similar mechanism to that of carboxylic acids. LiAlH4 first converts the ester into an aldehyde, and then the aldehyde is reduced to a primary alcohol.
| 5. Can LiAlH4 reduce nitriles to primary amines? |  |
Ans. Yes, LiAlH4 can reduce nitriles to primary amines. The reduction involves the conversion of the nitrile into an imine intermediate, which is then reduced to give the primary amine.
Related Searches




















