UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Di-isobutyl Aluminum Hydride (DIBAL)
Di-isobutyl Aluminum Hydride (DIBAL) | Chemistry Optional Notes for UPSC PDF Download
Introduction
- DIBAL (also known as DIBAL-H or DIBAH) is a strong, bulky reducing agent.
- It’s most useful for the reduction of esters to aldehydes.
- Unlike lithium aluminum hydride, it will not reduce the aldehyde further provided that only one equivalent is added and the reaction mixture is kept cold.
- It will also reduce other carbonyl compounds such as amides, aldehydes, ketones, and nitriles.
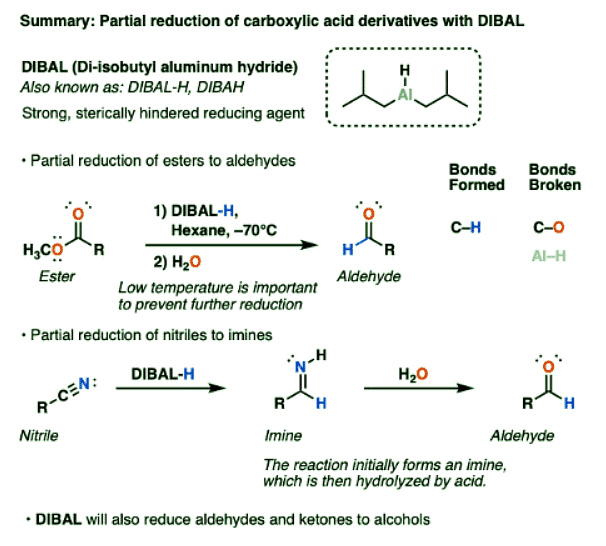
Structure of Di-isobutyl Aluminum Hydride (DIBAL)
- DIBAL is a strong, bulky reducing agent. Reactivity-wise, it bears a lot of similarity to lithium aluminum hydride.
- Unlike LiAlH4 which can (in theory) deliver four equivalents of hydride, DIBAL bears only a single Al-H bond.
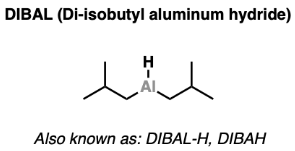
- This makes the stoichiometry of its reactions much easier to control.
Reduction Of Esters To Aldehydes With DIBAL
- The most notable reaction of DIBAL is the reduction of esters to aldehydes. Unlike lithium aluminum hydride (LiAlH4), which reduces esters to primary alcohols, reductions with DIBAL can stop at the aldehyde stage if the temperature is kept very low.
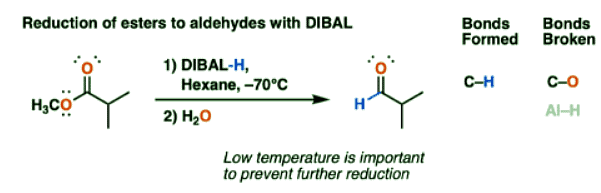
- The advantage of DIBAL here is that it is more efficient. If we use LiAlH4, we obtain a primary alcohol, which we would then have to oxidize up to the aldehyde using a reagent such as PCC, Dess-Martin periodinane, or the Swern oxidation.
Question for Di-isobutyl Aluminum Hydride (DIBAL)Try yourself: What is the main advantage of using DIBAL compared to LiAlH4 in the reduction of esters to aldehydes?View Solution
Reduction Of Esters To Aldehydes By Di-Isobutyl Aluminum Hydride: Mechanism
- The mechanism for reduction of esters to aldehydes with DIBAL is roughly similar to the familiar addition-elimination mechanism of nucleophilic acyl substitution, with a slightly modified first step.
- DIBAL-H is neutral, and aluminum, being in the same column of the periodic table as boron, has an empty p-orbital. This makes it a Lewis acid.
- The carbonyl oxygen of esters is a Lewis base. So the first step is coordination of the Lewis basic carbonyl oxygen to the Lewis acidic aluminum, to give a species with a negative formal charge on aluminum.
- Then comes nucleophilic addition of a hydride (H-) to the carbonyl carbon alongside breakage of the C-O pi bond (form C-H, break C-O pi).
- This forms a new tetrahedral intermediate which is essentially a hemiacetal coordinated to aluminum.
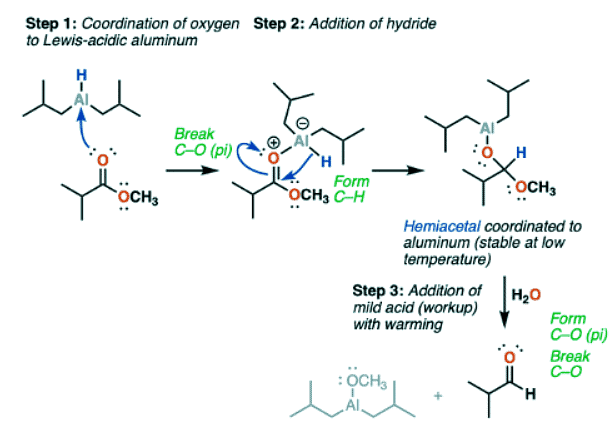
- If the temperature is kept low (the convenient dry ice-acetone bath of –78°C is often quoted) , a reaction quenched with acid at this stage will give a neutral hemiacetal which quickly loses ROH to give an aldehyde.
Reduction Of Nitriles To Imines With DIBAL (And Subsequent Hydrolysis To Aldehydes)
DIBAL will also do partial reductions of nitriles to imines. The imines are then hydrolyzed to aldehydes upon addition of water. In this respect DIBAL again differs from LiAlH4, which will reduce nitriles all the way to primary amines.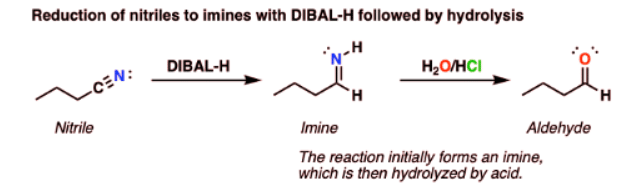
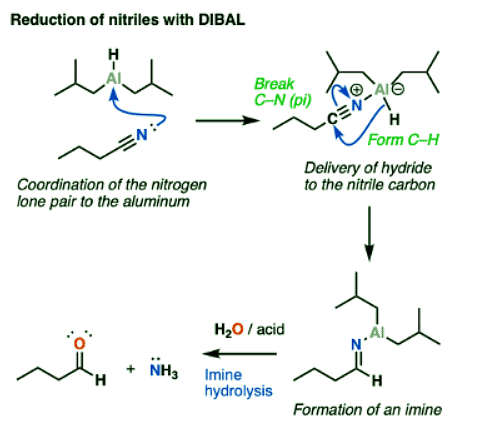
Reduction Of Nitriles To Imines: Mechanism
- Reduction of nitriles follows a similar mechanism to that for the reduction of esters. Coordination of the Lewis-basic nitrile nitrogen to aluminum is followed by delivery of hydride to the nitrile carbon (form C-H, break C-N (pi) ). This is another example of an addition mechanism.
- From this point, quenching the reaction with water results in protonation of the nitrogen and then hydrolysis of the imine to give an aldehyde.
Reduction Of Ketones And Aldehydes To Alcohols With Di-IsobutylAluminum Hydride (DIBAL)
- Although not often used this way, it’s worth noting that DIBAL can do all the reductions that NaBH4 does, so ketones and aldehydes are reduced to secondary and primary alcohols, respectively.
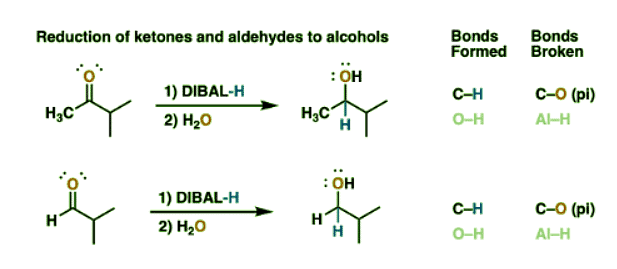
- What this means is that DIBAL will not selectively reduce an ester in the presence of an unprotected aldehyde or ketone – those groups will get reduced too.
Summary: DIBAL
- DIBAL is a useful reagent for the partial reduction of carboxylic acid derivatives. A successful DIBAL reduction of an ester to an aldehyde will save an extra step relative to LiAlH4 followed by oxidation.
- Likewise, DIBAL reduction of nitriles gives us a route to aldehydes (after hydrolysis) that is not available through reduction with LiAlH4, which completely reduces nitriles to amines.
The document Di-isobutyl Aluminum Hydride (DIBAL) | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Di-isobutyl Aluminum Hydride (DIBAL) - Chemistry Optional Notes for UPSC
| 1. What is the structure of Di-isobutyl Aluminum Hydride (DIBAL)? |  |
Ans. The structure of Di-isobutyl Aluminum Hydride (DIBAL) is (CH3)2CHCH2AlH2.
| 2. How does DIBAL reduce esters to aldehydes? |  |
Ans. DIBAL reduces esters to aldehydes by adding one hydride (H-) to the carbonyl oxygen of the ester, followed by a second hydride transfer to the carbonyl carbon, resulting in the formation of an aldehyde.
| 3. What is the mechanism of the reduction of esters to aldehydes by DIBAL? |  |
Ans. The mechanism involves the coordination of the ester to the aluminum center of DIBAL, followed by hydride transfer from DIBAL to the carbonyl oxygen of the ester. This is followed by another hydride transfer from DIBAL to the carbonyl carbon, resulting in the formation of an aldehyde.
| 4. How does DIBAL reduce nitriles to imines? |  |
Ans. DIBAL reduces nitriles to imines by adding one hydride (H-) to the nitrile carbon, resulting in the formation of an imine.
| 5. What is the mechanism of the reduction of nitriles to imines by DIBAL? |  |
Ans. The mechanism involves the coordination of the nitrile to the aluminum center of DIBAL, followed by hydride transfer from DIBAL to the nitrile carbon. This results in the formation of an imine, which can then undergo hydrolysis to form an aldehyde.
Related Searches




















