Mass Spectrometry in Biological - Time-of-flight (TOF) Instruments | Chemistry Optional Notes for UPSC PDF Download
Mass Spectrometry in Biological - Time-of-flight (TOF) Instruments
MS analyses of sensitive biological samples rarely use magnetic sector ionization. Instead, they typically use either electrospray ionization (ESI) or matrix-assisted laser desorption ionization (MALDI), typically linked to a time-of-flight (TOF) mass analyzer. Both ESI and MALDI are soft ionization methods that produce charged molecules with little fragmentation, even with sensitive biological samples of very high molecular weight.
In an ESI source, as a sample solution exits the tube, it is subjected to a high voltage that causes the droplets to become charged. The sample molecules gain one or more protons from charged solvent molecules in the droplet. The volatile solvent quickly evaporates, giving variably protonated sample molecules (M + Hnn+). In a MALDI source, the sample is adsorbed onto a suitable matrix compound, such as 2,5-dihydroxybenzoic acid, which is ionized by a short burst of laser light. The matrix compound then transfers the energy to the sample and protonates it, forming M + Hnn+ ions.
Following ion formation, the variably protonated sample molecules are electrically focused into a small packet with a narrow spatial distribution, and the packet is given a sudden kick of energy by an accelerator electrode. As each molecule in the packet is given the same energy, E = mv2/2, it begins moving with a velocity that depends on the square root of its mass, 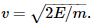 Lighter molecules move faster, and heavier molecules move slower. The analyzer itself—the drift tube—is simply an electrically grounded metal tube inside which the different charged molecules become separated as they move at different velocities and take different amounts of time to complete their flight.
Lighter molecules move faster, and heavier molecules move slower. The analyzer itself—the drift tube—is simply an electrically grounded metal tube inside which the different charged molecules become separated as they move at different velocities and take different amounts of time to complete their flight.
The Time of Flight technique is considerably more sensitive than the magnetic sector alternative, and protein samples of up to 100 kilodaltons (100,000 amu) can be separated with a mass accuracy of 3 ppm. Figure 12.16 shows a MALDI–TOF spectrum of chicken egg-white lysozyme, MW = 14,306.7578 daltons. Biochemists generally use the unit dalton, abbreviated Da, instead of amu, although the two are equivalent (1 dalton = 1 amu).
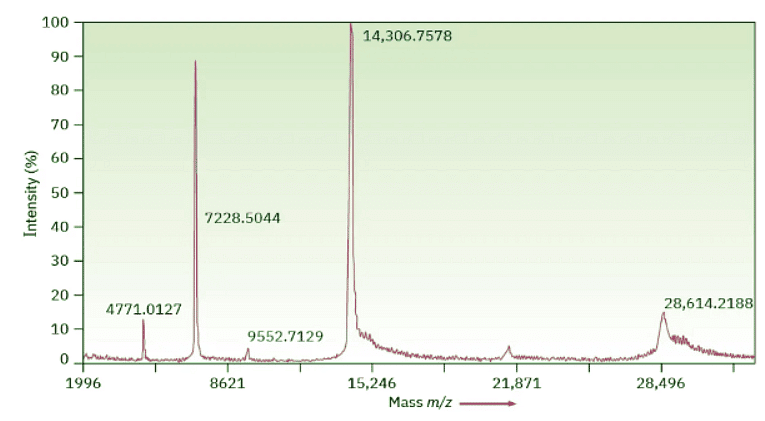 Figure 12.16: MALDI–TOF mass spectrum of chicken egg-white lysozyme. The peak at 14,306.7578 daltons (amu) is due to the monoprotonated protein, M + H+, and the peak at 28,614.2188 daltons is due to an impurity formed by dimerization of the protein. Other peaks at lower m/z values are various protonated species, M + Hnn+.
Figure 12.16: MALDI–TOF mass spectrum of chicken egg-white lysozyme. The peak at 14,306.7578 daltons (amu) is due to the monoprotonated protein, M + H+, and the peak at 28,614.2188 daltons is due to an impurity formed by dimerization of the protein. Other peaks at lower m/z values are various protonated species, M + Hnn+.
FAQs on Mass Spectrometry in Biological - Time-of-flight (TOF) Instruments - Chemistry Optional Notes for UPSC
| 1. What is mass spectrometry and how is it used in biological research? |  |
| 2. How does a time-of-flight (TOF) mass spectrometer work? |  |
| 3. What are the advantages of using a time-of-flight (TOF) mass spectrometer in biological research? |  |
| 4. How can time-of-flight (TOF) mass spectrometry be used in proteomics research? |  |
| 5. What are some applications of time-of-flight (TOF) mass spectrometry in biological research? |  |














