UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Ziegler–Natta Catalysts and Polymer Stereochemistry
Ziegler–Natta Catalysts and Polymer Stereochemistry | Chemistry Optional Notes for UPSC PDF Download
Regioselectivity in Polymerization: Symmetrical vs. Monosubstituted Monomers
- Symmetrical monomers such as ethylene and tetrafluoroethylene can join together in only one way. Monosubstituted monomers, on the other hand, may join together in two organized ways, described in the following diagram, or in a third random manner. Most monomers of this kind, including propylene, vinyl chloride, styrene, acrylonitrile and acrylic esters, prefer to join in a head-to-tail fashion, with some randomness occurring from time to time. The reasons for this regioselectivity will be discussed in the synthetic methods section.
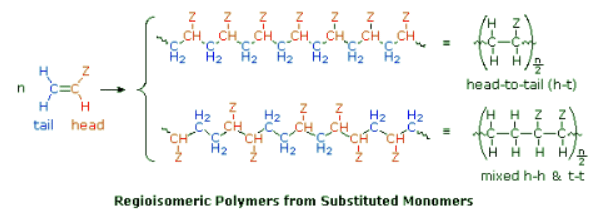
- If the polymer chain is drawn in a zig-zag fashion, as shown above, each of the substituent groups (Z) will necessarily be located above or below the plane defined by the carbon chain. Consequently we can identify three configurational isomers of such polymers. If all the substituents lie on one side of the chain the configuration is called isotactic. If the substituents alternate from one side to another in a regular manner the configuration is termed syndiotactic. Finally, a random arrangement of substituent groups is referred to as atactic. Examples of these configurations are shown here.
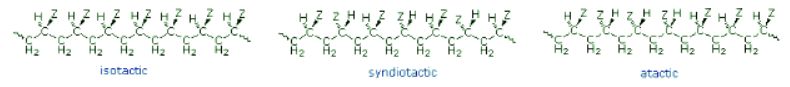
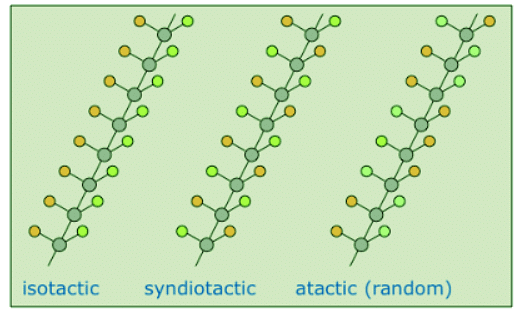
- Many common and useful polymers, such as polystyrene, polyacrylonitrile and poly(vinyl chloride) are atactic as normally prepared. Customized catalysts that effect stereoregular polymerization of polypropylene and some other monomers have been developed, and the improved properties associated with the increased crystallinity of these products has made this an important field of investigation. The following values of Tg have been reported.
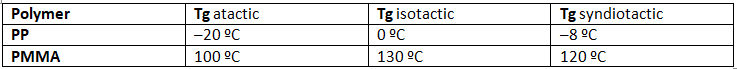
- The properties of a given polymer will vary considerably with its tacticity. Thus, atactic polypropylene is useless as a solid construction material, and is employed mainly as a component of adhesives or as a soft matrix for composite materials. In contrast, isotactic polypropylene is a high-melting solid (ca. 170 ºC) which can be molded or machined into structural components.
Question for Ziegler–Natta Catalysts and Polymer StereochemistryTry yourself: Which type of monomers prefer to join in a head-to-tail fashion with some randomness occurring from time to time?View Solution
Ziegler-Natta Catalytic Polymerization
- An efficient and stereospecific catalytic polymerization procedure was developed by Karl Ziegler (Germany) and Giulio Natta (Italy) in the 1950's. Their findings permitted, for the first time, the synthesis of unbranched, high molecular weight polyethylene (HDPE), laboratory synthesis of natural rubber from isoprene, and configurational control of polymers from terminal alkenes like propene (e.g. pure isotactic and syndiotactic polymers). In the case of ethylene, rapid polymerization occurred at atmospheric pressure and moderate to low temperature, giving a stronger (more crystalline) product (HDPE) than that from radical polymerization (LDPE). For this important discovery these chemists received the 1963 Nobel Prize in chemistry.
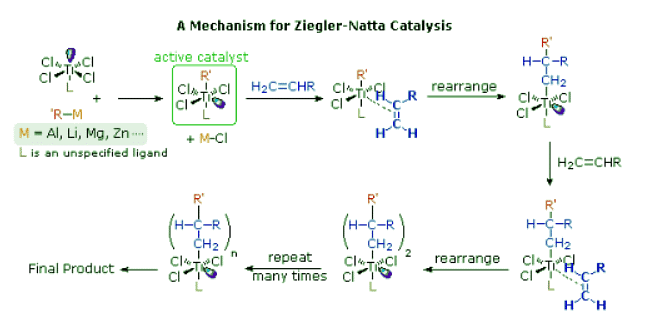
- Ziegler-Natta catalysts are prepared by reacting certain transition metal halides with organometallic reagents such as alkyl aluminum, lithium and zinc reagents. The catalyst formed by reaction of triethylaluminum with titanium tetrachloride has been widely studied, but other metals (e.g. V & Zr) have also proven effective. The following diagram presents one mechanism for this useful reaction. Others have been suggested, with changes to accommodate the heterogeneity or homogeneity of the catalyst. Polymerization of propylene through action of the titanium catalyst gives an isotactic product; whereas, a vanadium based catalyst gives a syndiotactic product.
Question for Ziegler–Natta Catalysts and Polymer StereochemistryTry yourself: Which type of monomers can join together in only one way?View Solution
The document Ziegler–Natta Catalysts and Polymer Stereochemistry | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Ziegler–Natta Catalysts and Polymer Stereochemistry - Chemistry Optional Notes for UPSC
| 1. What is regioselectivity in polymerization and how does it relate to symmetrical and monosubstituted monomers? |  |
Ans. Regioselectivity in polymerization refers to the preference of a polymerization reaction to occur at a specific site on a monomer molecule. In the context of symmetrical and monosubstituted monomers, regioselectivity determines which carbon atom within the monomer is most likely to participate in the polymerization reaction. For symmetrical monomers, regioselectivity is usually low as both ends of the monomer are chemically equivalent and have similar reactivity. On the other hand, monosubstituted monomers have a higher regioselectivity as the substituent group influences the reactivity of the carbon atoms, leading to a preference for polymerization at the unsubstituted carbon atom.
| 2. What is the Ziegler-Natta catalytic polymerization and how does it work? |  |
Ans. The Ziegler-Natta catalytic polymerization is a method used to produce high-quality polymers with controlled molecular structures and stereochemistry. It involves the use of Ziegler-Natta catalysts, which are transition metal compounds supported on an inorganic carrier. The catalysts typically consist of a transition metal, such as titanium or zirconium, along with an organoaluminum compound as a co-catalyst.
In the Ziegler-Natta polymerization process, the catalyst activates the monomer molecules by coordinating them to the transition metal center. This coordination allows for controlled insertion of monomer units into the growing polymer chain. The stereospecificity of the catalyst determines the stereochemistry of the resulting polymer, leading to the formation of either isotactic, syndiotactic, or atactic polymers.
| 3. How do Ziegler-Natta catalysts influence polymer stereochemistry? |  |
Ans. Ziegler-Natta catalysts play a crucial role in determining the stereochemistry of the resulting polymer. The stereochemistry refers to the spatial arrangement of the monomer units along the polymer chain.
These catalysts exhibit stereospecificity, meaning they have a preference for incorporating monomer units in a specific spatial configuration. This preference is determined by the structure and coordination environment of the transition metal center in the catalyst. For example, catalysts based on titanium typically produce isotactic polymers, where the monomer units have the same spatial orientation along the polymer chain. Catalysts based on zirconium, on the other hand, tend to produce syndiotactic polymers, where the monomer units alternate in their spatial orientation along the chain.
| 4. What are some frequently asked questions about Ziegler-Natta catalytic polymerization? |  |
Ans.
1. How does the Ziegler-Natta catalytic polymerization differ from other polymerization methods?
2. What types of polymers can be produced using Ziegler-Natta catalysts?
3. How do Ziegler-Natta catalysts contribute to the control of molecular weight in polymerization reactions?
4. Can Ziegler-Natta catalysts be used for the polymerization of different monomers?
5. What are the advantages and limitations of using Ziegler-Natta catalysts in industrial polymer production?
| 5. How does the regioselectivity of monomers affect the properties of the resulting polymers? |  |
Ans. The regioselectivity of monomers can significantly influence the properties of the resulting polymers. When polymerization occurs preferentially at one end of a monomer, it leads to the formation of a polymer with a specific chemical structure and arrangement of functional groups. This, in turn, affects the polymer's physical and chemical properties, such as its solubility, melting point, and reactivity.
For example, in the case of monosubstituted monomers, where polymerization predominantly occurs at the unsubstituted carbon atom, the resulting polymers often have a higher density and improved thermal stability compared to their symmetrical counterparts. This is because the presence of the substituent group can hinder the packing of polymer chains, leading to a more tightly packed and higher density structure. Additionally, the presence of the substituent group can also affect the polymer's reactivity towards other chemicals or functional groups, making it useful for specific applications in industries such as materials science and engineering.
Related Searches















