UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Pinacol Rearrangement
Pinacol Rearrangement | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Introduction |

|
| The Pinacol Rearrangement |

|
| Mechanism Of The Pinacol Rearrangement |

|
| Pinacol Rearrangements With Ring Expansion |

|
| Summary |

|
Introduction
- The pinacol rearrangement is an acid-catalyzed rearrangement of 1,2-diols (vicinal diols)
- The acid serves to protonate one of the hydroxyl groups, which departs as water, giving a carbocation.
- Subsequently, a C-O pi bond is formed as a C-C bond migrates to the adjacent carbon.
- It resembles the familiar hydride shifts and alkyl shifts in that a less stable carbocation is converted to a more stable carbocation (albeit with the twist is that this “more stable carbocation” is actually the resonance form of a protonated ketone).
- Pinacol rearrangements can result in ring expansions
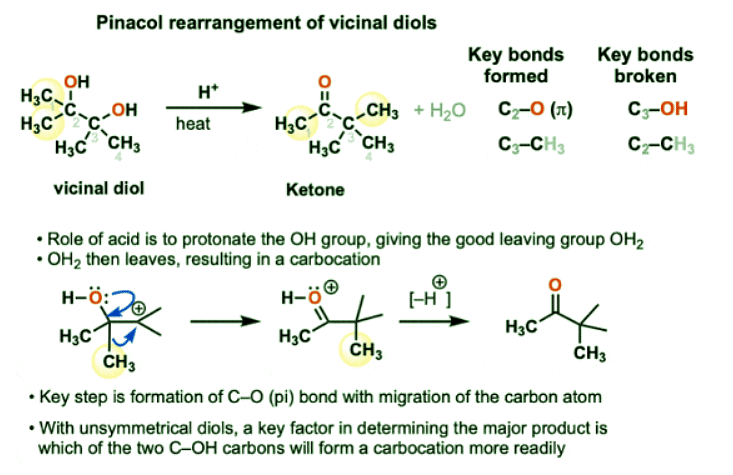
The Pinacol Rearrangement
- Pinacol is a pleasant-smelling 1,2-diol (“vicinal” diol) with the following structure:
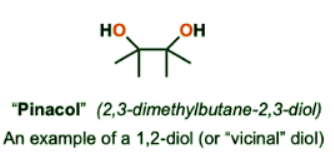
- When pinacol is treated with strong acid such as sulfuric acid (H2SO4), a new ketone is formed and a molecule of water is lost from the diol. Upon inspection, it can be seen that the carbon skeleton has undergone rearrangement:
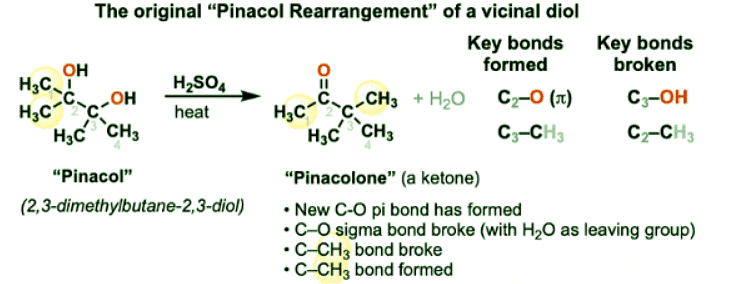
- The most important thing to note here is that one of the highlighted CH3 groups on C2 has moved to C3.
- This reaction has become known as the pinacol rearrangement, and it is a fairly common rearrangement of 1,2-diols, having first been observed as far back as 1860. [See Ref 1]
- It is a special case of our old friends the 1,2-hydride shift and the 1,2-alkyl shift (See article: Rearrangement Reactions – Hydride Shifts)
- The reaction can be performed with a wide variety of other vicinal diols. Here is another example:
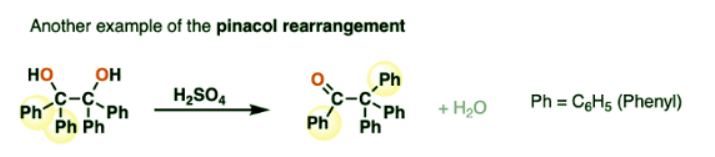
- In this case, a phenyl group migrates to the adjacent carbon to give a new ketone adjacent to a quaternary carbon.
Question for Pinacol Rearrangement
Try yourself:
Which of the following is true about the pinacol rearrangement?View Solution
Mechanism Of The Pinacol Rearrangement
- So how does this reaction work?
- If we think back to 1,2-hydride shifts and 1,2-alkyl shifts, we’ll recall that they involve formation of a carbocation followed by migration of H(-) or R(-) to generate a new carbocation. (See article: Rearrangement Reactions With Alkyl Shifts)
- So how might we generate a carbocation here from the vicinal diol and the acid?
- By protonating one of the alcohols, we generate its conjugate acid R-OH2(+)
- Since H2O is a much weaker base than HO(-), this molecule has a much better leaving group. Breakage of the C-O bond then generates a carbocation.
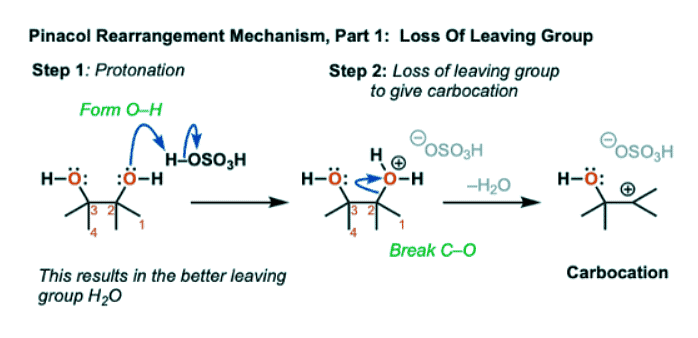
- Now, the key rearrangement step can occur, whereby a methyl group (CH3) can migrate from C-2 to C-3. The C2-CH3 bond breaks and the C3-CH3 bond forms, giving a new carbocation.
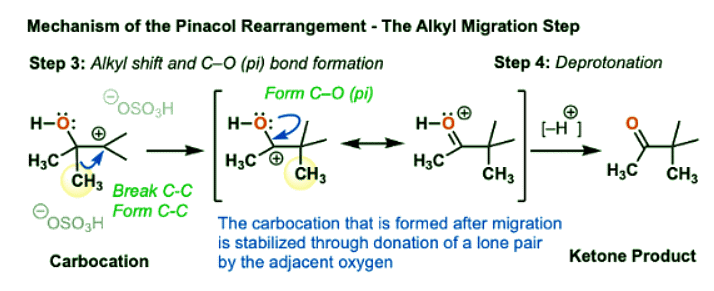
- Recall that rearrangements are generally favored when they result in a more stable carbocation. Is this true in this case?
- Actually, yes!
- If you look at the new carbocation that is formed, it can have its octet filled through donation of a lone pair by the adjacent oxygen, forming a new pi-bond in the process. (Note: this is often called, “pi-donation” and it’s extremely important).
- That is, this “carbocation” is actually just a resonance form of a protonated ketone!
- The final step is deprotonation of the protonated ketone with a weak base to give the neutral ketone.
- That’s it.
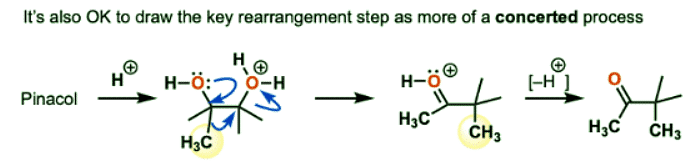
- Although I’ve shown the reaction as proceeding stepwise, it’s also perfectly acceptable to show it as occurring in a concerted fashion. Note 1.
Pinacol Rearrangements With Ring Expansion
- The pinacol rearrangement can also occur with ring expansion.
- That is, pinacol rearrangements of cyclic molecules can result in the formation of larger rings.
- This is fundamentally no different from the mechanism of a typical pinacol rearrangement.
 |
Download the notes
Pinacol Rearrangement
|
Download as PDF |
Download as PDF
Notes
- Note 1: Here is an example of showing the key rearrangement step as a concerted reaction. For more discussion on the plausibility of concerted versus stepwise pinacol rearrangements, see Ref 9.
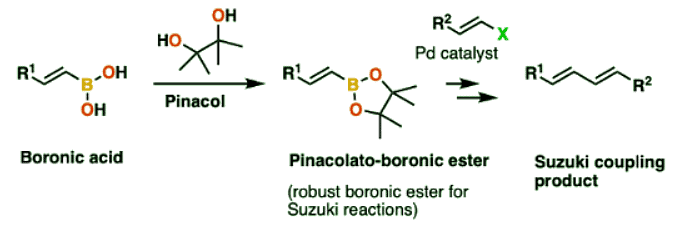
- Note 2: Pinacol itself is often encountered in the Suzuki reaction, since boronic esters with pinacol are highly stable and can be purified by column chromatography.
Summary
- The pinacol rearrangement is a special case of carbocation rearrangement where migration of a carbon is accompanied by formation of a new C-O (pi) bond. The product is a ketone.
- The pinacol rearrangement is promoted by the addition of acid which assists in loss of water as a leaving group.
- Make sure you can draw the mechanism of pinacol rearrangements that result in ring expansion!
Question for Pinacol Rearrangement
Try yourself:
Which of the following statements is true regarding the pinacol rearrangement?View Solution
The document Pinacol Rearrangement | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Pinacol Rearrangement - Chemistry Optional Notes for UPSC
| 1. What is the Pinacol Rearrangement? |  |
| 2. What is the mechanism of the Pinacol Rearrangement? |  |
Ans. The Pinacol Rearrangement involves the protonation of the oxygen atom in the pinacol, followed by migration of a hydrogen atom and rearrangement of the carbon skeleton. This is typically followed by deprotonation to form the final product.
| 3. Can the Pinacol Rearrangement result in ring expansion? |  |
Ans. Yes, the Pinacol Rearrangement can result in ring expansion. This occurs when the rearrangement leads to the formation of a larger ring system than the starting material. This is often observed in cyclic pinacols.
| 4. How is the Pinacol Rearrangement relevant to the UPSC exam? |  |
Ans. The Pinacol Rearrangement is a topic that is often covered in organic chemistry courses, which is a part of the UPSC exam syllabus. Understanding this reaction and its mechanism is important for students preparing for the exam.
| 5. What is the summary of the Pinacol Rearrangement? |  |
Ans. The Pinacol Rearrangement is a chemical reaction that involves the rearrangement of a pinacol to form a ketone or aldehyde. This reaction can result in ring expansion and is an important topic in organic chemistry for the UPSC exam.
Related Searches





















