Hydration of Alkenes - Addition of H₂O by Hydroboration | Chemistry Optional Notes for UPSC PDF Download
Hydration of Alkenes - Addition of H₂O by Hydroboration
In addition to the oxymercuration–demercuration method, which yields the Markovnikov product, a complementary method that yields the non-Markovnikov product is also useful. Discovered in 1959 by H.C. Brown at Purdue University and called hydroboration, the reaction involves addition of a B−H bond of borane, BH3, to an alkene to yield an organoborane intermediate, RBH2. Oxidation of the organoborane by reaction with basic hydrogen peroxide, H2O2, then gives an alcohol. For example: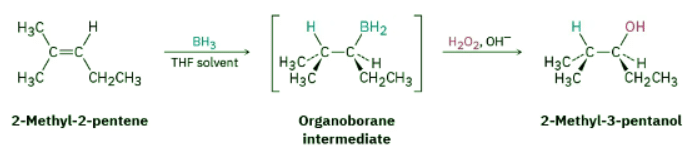
Borane is very reactive as a Lewis acid because the boron atom has only six electrons in its valence shell rather than an octet. In tetrahydrofuran solution, BH3 accepts an electron pair from a solvent molecule in a Lewis acid–base reaction to complete its octet and form a stable BH3–THF complex.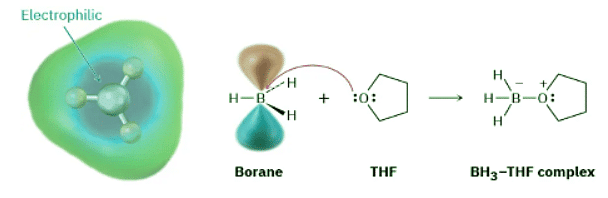
When an alkene reacts with BH3 in THF solution, rapid addition to the double bond occurs three times and a trialkylborane, R3B, is formed. For example, 1 molar equivalent of BH3 adds to 3 molar equivalents of cyclohexene to yield tricyclohexylborane. When tricyclohexylborane is then treated with aqueous hydrogen H2O2 in basic solution, an oxidation takes place. The three C−B bonds are broken, −OH groups bond to the three carbons, and 3 equivalents of cyclohexanol are produced. The net effect of the two-step hydroboration–oxidation sequence is hydration of the alkene double bond.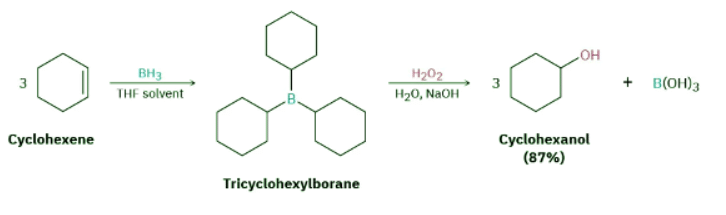
One of the features that makes the hydroboration reaction so useful is the regiochemistry that results when an unsymmetrical alkene is hydroborated. For example, hydroboration–oxidation of 1-methylcyclopentene yields trans-2-methylcyclopentanol. In this process, boron and hydrogen add to the alkene from the same face of the double bond—that is, with syn stereochemistry, the opposite of anti—with boron attaching to the less highly substituted carbon. During the oxidation step, the boron is replaced by an −OH with the same stereochemistry, resulting in an overall syn non-Markovnikov addition of water. This stereochemical result is particularly useful because it is complementary to the Markovnikov regiochemistry observed for oxymercuration–demercuration.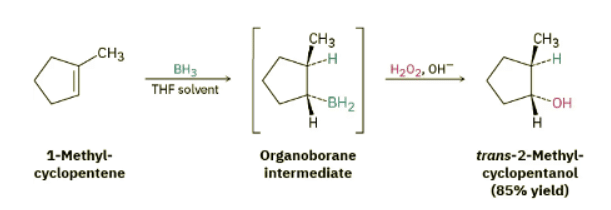
Why does alkene hydroboration take place with syn, non-Markovnikov regiochemistry to yield the less highly substituted alcohol? Hydroboration differs from many other alkene addition reactions in that it occurs in a single step without a carbocation intermediate (Figure 8.6). Because the C−H and C−B bonds form at the same time and from the same face of the alkene, syn stereochemistry results. Non-Markovnikov regiochemistry occurs because attachment of boron is favored at the less sterically crowded carbon atom of the alkene.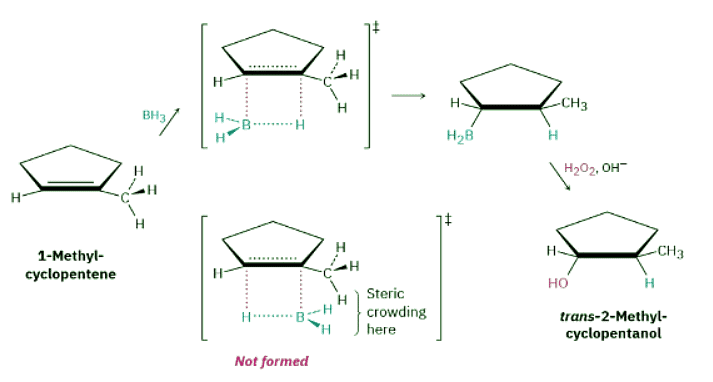 Figure 8.6: Mechanism of alkene hydroboration. The reaction occurs in a single step in which the C−H and C−B bonds form at the same time and on the same face of the double bond. The lower energy, more rapidly formed transition state is the one with less steric crowding, leading to non-Markovnikov regiochemistry.
Figure 8.6: Mechanism of alkene hydroboration. The reaction occurs in a single step in which the C−H and C−B bonds form at the same time and on the same face of the double bond. The lower energy, more rapidly formed transition state is the one with less steric crowding, leading to non-Markovnikov regiochemistry.
Solved Examples
Example 1: Predicting the Products of a Hydration Reaction
What products would you obtain from reaction of 2-methyl-2-pentene with:
(a) BH3, followed by H2O2, OH−
(b) Hg(OAc)2, followed by NaBH4
Ans: Strategy: When predicting the product of a reaction, you have to recall what you know about the kind of reaction being carried out and apply that knowledge to the specific case you’re dealing with. In the present instance, recall that the two methods of hydration—hydroboration–oxidation and oxymercuration–demercuration—give complementary products. Hydroboration–oxidation occurs with syn stereochemistry and gives the non-Markovnikov addition product; oxymercuration–demercuration gives the Markovnikov product.
Solution: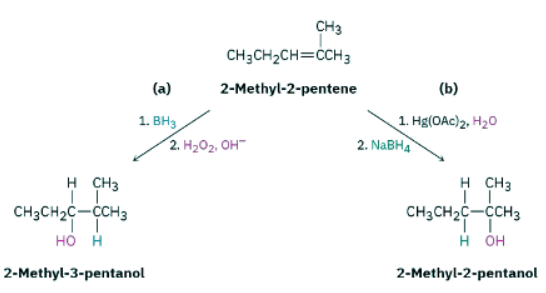
Example 2: Synthesizing an Alcohol
How might you prepare the following alcohol?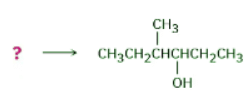 Ans: Strategy: Problems that require the synthesis of a specific target molecule should always be worked backward. Look at the target, identify its functional group(s), and ask yourself, “What are the methods for preparing that functional group?” In the present instance, the target molecule is a secondary alcohol (R2CHOH), and we’ve seen that alcohols can be prepared from alkenes by either hydroboration–oxidation or oxymercuration–demercuration. The −OH-bearing carbon in the product must have been a double-bond carbon in the alkene reactant, so there are two possibilities here: 4-methyl-2-hexene and 3-methyl-3-hexene.
Ans: Strategy: Problems that require the synthesis of a specific target molecule should always be worked backward. Look at the target, identify its functional group(s), and ask yourself, “What are the methods for preparing that functional group?” In the present instance, the target molecule is a secondary alcohol (R2CHOH), and we’ve seen that alcohols can be prepared from alkenes by either hydroboration–oxidation or oxymercuration–demercuration. The −OH-bearing carbon in the product must have been a double-bond carbon in the alkene reactant, so there are two possibilities here: 4-methyl-2-hexene and 3-methyl-3-hexene.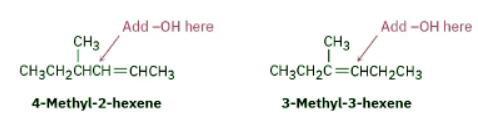
4-Methyl-2-hexene has a disubstituted double bond, RCH═CHR', and will probably give a mixture of two alcohols with either hydration method since Markovnikov’s rule does not apply to symmetrically substituted alkenes. 3-Methyl-3-hexene, however, has a trisubstituted double bond, and should give only the desired product on non-Markovnikov hydration using the hydroboration–oxidation method.
Solution: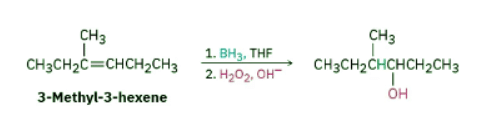
FAQs on Hydration of Alkenes - Addition of H₂O by Hydroboration - Chemistry Optional Notes for UPSC
| 1. What is the process of hydration of alkenes? |  |
| 2. How is hydroboration used in the hydration of alkenes? |  |
| 3. What is the significance of hydroboration in the hydration of alkenes? |  |
| 4. What are the conditions required for the hydroboration process in the hydration of alkenes? |  |
| 5. What are the products formed in the hydration of alkenes by hydroboration? |  |




















