UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > E1 Rxn Rearrangements & SN1 E1 Comparison
E1 Rxn Rearrangements & SN1 E1 Comparison | Chemistry Optional Notes for UPSC PDF Download
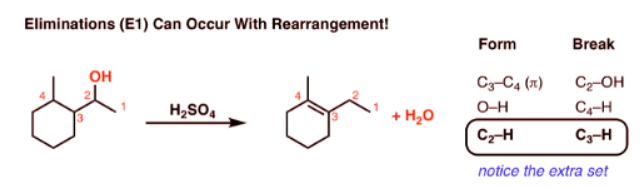
What’s Weird About This Elimination Reaction?
- One last (weird) reaction to show you with regard to elimination reactions. Can you see what’s weird about it?

- How did that double bond get over there? Normally when elimination occurs, we remove a hydrogen from the carbon adjacent to the leaving group. But here, something extra has taken place.
Elimination (E1) With Rearrangement: Hydride Shift
- Let’s look at all the bonds that form and the bonds that break so we can track down exactly what happens:

- Notice how it differs from a typical elimination reaction? Sure, we’re forming C-C (π), and breaking C-H and C-OH, but we have an extra C-H that forms and an extra C-H that breaks.
- This is a sure sign of a rearrangement step!
- So what’s going on here?
- Well, we start by protonating the alcohol. This allows for water to leave in the next step, which is going to form a carbocation.
- Here’s the thing: the carbocation is secondary, and we’re adjacent to a tertiary carbon. So if the hydrogen (and its pair of electrons) were to migrate from C3 in our example to C-2, we’d now have a tertiary carbocation, which is more stable.
- Then, a base (water in this example) could remove C-H, forming the more substituted alkene (the Zaitsev product in this case). And that’s how the alkene ends up there.

- OK. So that’s one mystery solved.
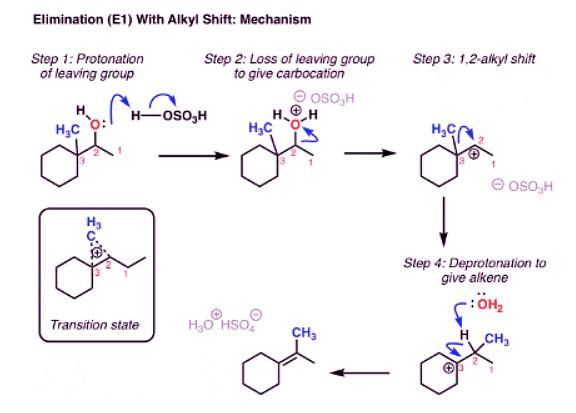
Elimination (E1) With Rearrangement: Alkyl Shift
- You might remember that these types of rearrangements can occur in SN1 reactions too. And if you read that post, you might recall that in addition to shifts of hydrogen (“hydride”, because there’s a pair of electrons attached) we can also have alkyl shifts. Here’s a final example.

- This pretty much does it for elimination reactions.
Question for E1 Rxn Rearrangements & SN1 E1 Comparison
Try yourself:
What is the unusual feature of the elimination reaction described in the passage?View Solution
Comparing the E1 vs SN1 Reactions
Alcohols Don’t Undergo Elimination Reactions Until OH Is Converted To A Better Leaving Group
- Imagine you’re starting with the alcohol on the left and you’d like to get to the alkene on the right.

- What bonds are formed and broken here? We’re forming C-C (π), we’re breaking C-H, and we’re breaking C-OH. It’s an elimination reaction.
- Notice a problem here? We need to have HO(-) as a leaving group. If you’ll recall, strong bases [like HO(-) ] are terrible leaving groups – which makes the E1 pathway unlikely. (See article: What Makes A Good Leaving Group)
- So what if we tried to use a strong base, maybe trying to promote an E2 reaction? Well, that would be even worse – we’d likely deprotonate OH before the C-H, and you can imagine that we’d have to have O(2-) as a leaving group here. Not good!
- That means that the reaction, as written, is very unlikely to happen.
 |
Download the notes
E1 Rxn Rearrangements & SN1 E1 Comparison
|
Download as PDF |
Download as PDF
Adding Acid To Alcohols Results In A Better Leaving Group
- Yet, there is something very simple that we can do to make this reaction work. We’d need to have a better leaving group (a weaker base). How can we do this?
- Add acid!

- If we add a strong acid, we turn OH into H2O+, the conjugate acid is a better leaving group. Now, water can leave, forming a carbocation; and then a base can break the C-H bond, forming the alkene.
- Notice that this is now a classic E1 reaction. The rate is going to be dependent on the stability of the carbocation. This one is tertiary, so it should proceed at a reasonably high rate.
- A question arises here. What’s going to act as the base? As it stands, a C-H bond adjacent to a carbocation has an extremely high acidity (at least below -2, if you follow pKa). That means that just about any weak base (water, or the conjugate base of the acid) is sufficient to deprotonate the carbon. It’s possible that more than one species can act as a base here. I’ve shown water removing the proton, but it’s not unreasonable to show the conjugate base of the acid removing the proton in most circumstances.
When Elimination And Substitution Compete: E1 vs SN1
- Now comes one of the things about organic chemistry that often causes trouble for students. For one of the first times in our discussions here, we’re dealing with a situation where we can have competing reactions.
- Let’s back up. The E1 reaction goes through a carbocation, correct? Well, if you’ll recall, so does the SN1 reaction.
- We’ve already seen examples where a carbocation was formed from an alcohol by adding a strong acid like HCl, HBr, or HI, and we ended up with the alkyl halide.
- Why? The halide ions (Cl- , Br-, I- ) are decent nucleophiles under the reaction conditions.
- So how can we stack the deck in favor of the E1 process?
- Use a strong acid with a conjugate base that is a poor nucleophile.
- The usual choice is H2SO4. The HSO4(-) ion is a relatively poor nucleophile due to the negative charge of the oxygen being distributed throughout the molecule (resonance). Two other acids you might see for this purpose are p-toluenesulfonic acid (p-TsOH), which is essentially a cousin of H2SO4, and phosphoric acid (H3PO4).

Question for E1 Rxn Rearrangements & SN1 E1 Comparison
Try yourself:
Which of the following is true regarding the E1 and SN1 reactions?View Solution
In Order To Get E1 To Predominate vs SN1 In The Reaction Of Alcohols Use H2SO4, TsOH, or H3PO4 (And Heat)
- In summary, if you’d like E1 to predominate over SN1: choose an acid with a weakly nucleophilic counterion [H2SO4, TsOH, or H3PO4], and heat.
- If you’d like SN1 to predominate over E1, choose an acid like HCl, HBr, or HI.
The document E1 Rxn Rearrangements & SN1 E1 Comparison | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on E1 Rxn Rearrangements & SN1 E1 Comparison - Chemistry Optional Notes for UPSC
| 1. What is the mechanism of the E1 elimination reaction? |  |
| 2. How does a hydride shift occur in the E1 elimination reaction? |  |
Ans. A hydride shift occurs in the E1 elimination reaction when a hydrogen atom shifts from one carbon to another, leading to the formation of a more stable carbocation. This rearrangement is observed when the migration of a hydrogen atom can form a more substituted and more stable carbocation intermediate.
| 3. What is the difference between an E1 and an SN1 reaction? |  |
Ans. The E1 and SN1 reactions are both unimolecular reactions that proceed via a carbocation intermediate. The key difference between the two is that the E1 reaction involves the elimination of a leaving group and the formation of a double bond, while the SN1 reaction involves the substitution of a leaving group with a nucleophile.
| 4. Why don't alcohols undergo elimination reactions until the hydroxyl group is converted into a better leaving group? |  |
Ans. Alcohols, in their original form, do not undergo elimination reactions because the hydroxyl group (OH) is a poor leaving group. In order for an alcohol to undergo elimination, the hydroxyl group must be converted into a better leaving group, typically by converting it into a more stable and more electronegative group, such as a halogen.
| 5. How does adding acid to alcohols result in a better leaving group? |  |
Ans. Adding acid to alcohols can result in the protonation of the hydroxyl group, converting it into a better leaving group. The protonated alcohol (oxonium ion) is more stable and can easily dissociate, forming a carbocation intermediate, which can then undergo elimination reactions. The addition of acid increases the electrophilicity of the hydroxyl group, making it more likely to leave the molecule.
Related Searches