Ethers: Properties, Preparation and Reactions | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| What are Ethers? |

|
| Physical Properties Of Ethers |

|
| Methods of Preparation of Ether |

|
| Chemical Reactions of Ethers |

|
| Uses of Ethers |

|
| Solved Examples of Ethers |

|
What are Ethers?
When you replace a hydrogen atom in a hydrocarbon with an alkoxy or aryloxy group (R–O/Ar–O), it gives rise to a different group of compounds called 'ethers'.
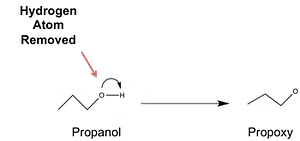 Formation of EtherThe general formula of ether is given as R-O-R, R-O-R’, R-O-Ar, or Ar-O-Ar where R represents an alkyl group and Ar represents an aryl group.
Formation of EtherThe general formula of ether is given as R-O-R, R-O-R’, R-O-Ar, or Ar-O-Ar where R represents an alkyl group and Ar represents an aryl group.
 General Formula of Ether
General Formula of Ether
Another way to think about ethers is that they are formed by swapping the hydrogen atom in the hydroxyl group of an alcohol or phenol with an alkyl or aryl group.
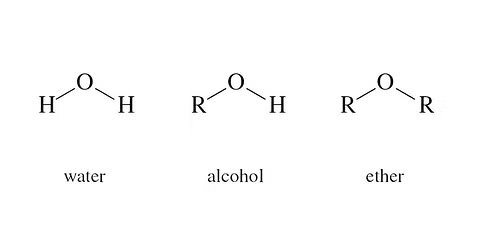 The Difference between Water, Alcohol, and Ether
The Difference between Water, Alcohol, and Ether
Classification of Ether
Depending on the type of the alkyl or aryl groups attached to the oxygen atom in ether, it can be classified into two types.
- Symmetrical ether: Also known as the simple ether, the alkyl or the aryl group attached to either side of the oxygen atoms are the same. Examples are CH3OCH, C2H5OC2H5, etc.
- Unsymmetrical ether: Also known as the mixed either, the alkyl or the aryl group attached to either side of the oxygen atoms, are not the same. Examples are CH3OC2H5, C2H5OC6H5, etc.
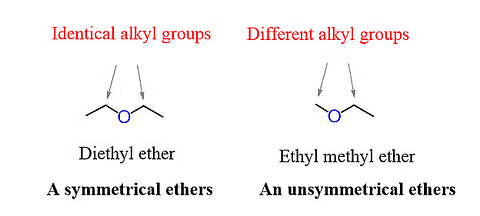 Symmetrical and Unsymmetrical Ethers
Symmetrical and Unsymmetrical Ethers
Examples of Ether
Ethers are a diverse group of organic compounds. Here are a few examples:
1. Dimethyl Ether (DME): CH3-O-CH3
2. Diethyl Ether: CH3-CH2-O-CH2-CH3
3. Methyl Propyl Ether: CH3-O-CH2-CH2-CH3
4. Anisole: CH3-O-C6H5
5. Ethyl Methyl Ether: CH3-CH2-O-CH3
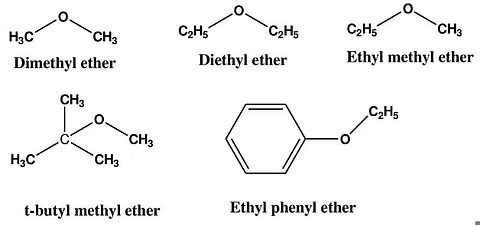 Common Examples of Ether
Common Examples of Ether
IUPAC Nomenclature of Ether (Alkoxy Alkane)
The common names of ethers follow a simple pattern. They are created by listing the names of alkyl/aryl groups as separate words in alphabetical order and then adding the word 'ether' at the end. For instance, CH3OC2H5 is called ethyl methyl ether.
When both alkyl groups in an ether are identical, we add the prefix 'di' before the alkyl group. For instance, C2H5OC2H5 is named diethyl ether.
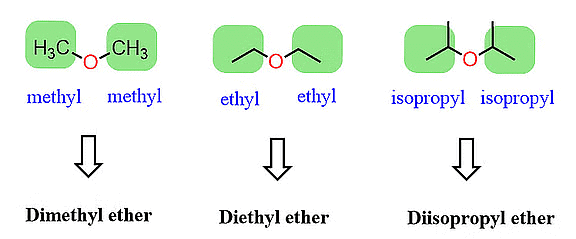 Traditional Nomenclature of Ether
Traditional Nomenclature of Ether
IUPAC's rules for naming ethers differ from traditional nomenclature. In IUPAC naming, the parent hydrocarbon is identified as the substituent group with more carbon atoms, while the other group connected to the oxygen atom is denoted with the prefix "oxy." For instance, CH3OC2H5 is designated as 1-Methoxy ethane.
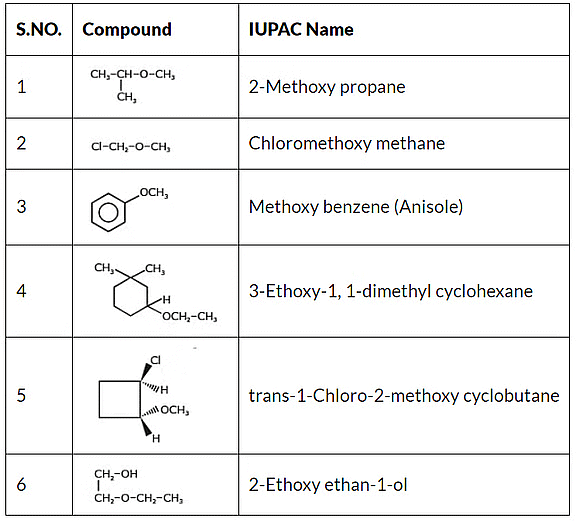 IUPAC Nomenclature of Ether
IUPAC Nomenclature of Ether
Important Tip: Naming of Ethers is one of the most asked questions in exams.
Physical Properties Of Ethers
- Physical State: Dimethyl ether and ethyl methyl ether exhibit gaseous states at room temperature. Their lower homologues, which share a colorless and pleasantly fragrant profile, are volatile liquids with the characteristic ether smell.
- Dipole Moment: The C-O-C bond angle, not aligning perfectly at 180°, results in the non-cancellation of dipole moments in the two C-O bonds. Consequently, ethers possess a modest net dipole moment.
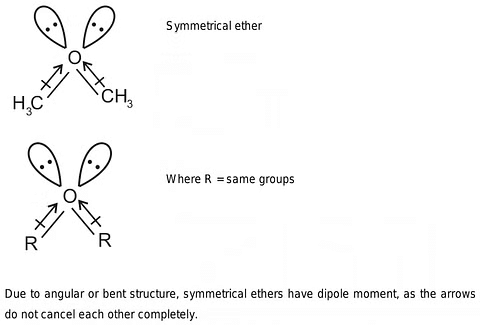 Dipole Moment of Ethers
Dipole Moment of Ethers
- Boiling Point: While the boiling points of ether molecules are comparable to alkanes, they are significantly lower than those of alcohols with similar molecular masses. This disparity is attributed to the absence of hydrogen bonding in ethers, a feature present in alcohols.
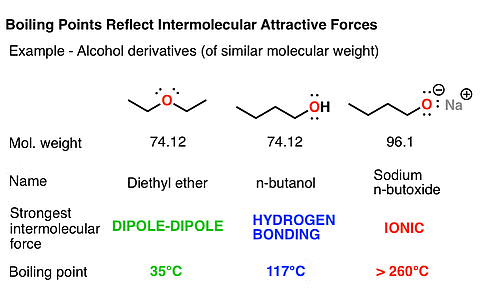 Comparison of Boiling Point of Ether with Alcohols
Comparison of Boiling Point of Ether with Alcohols
- Solubility: Ethers share a solubility pattern with alcohols of similar molecular masses. Their ability to dissolve in water stems from the oxygen atoms forming hydrogen bonds with water molecules. Solubility diminishes with an increase in carbon atoms, as a higher hydrocarbon content reduces the tendency for hydrogen bond formation.
- Polarity: Ethers exhibit lower polarity compared to esters, alcohols, or amines due to the hindered participation of the oxygen atom in hydrogen bonding caused by bulky alkyl groups flanking both sides of the oxygen atom. However, ethers are more polar than alkenes.
- Hybridization: In ethers, the oxygen atom adopts sp3 hybridization with a bond angle of 109.50°.
Methods of Preparation of Ether
(1) Williamson synthesis:
In laboratories, Williamson synthesis stands out as a crucial approach for crafting both symmetrical and asymmetrical ethers. This method involves the reaction of an alkyl halide with sodium alkoxide, resulting in the synthesis of ethers.
In this reaction, an alkoxide ion engages in an SN2 attack on an alkyl halide. Alkoxides, recognized for their potent basic nature, consistently react with alkyl halides and readily participate in elimination reactions.
Williamson synthesis demonstrates increased efficiency when applied to primary alkyl halides.
General reaction:
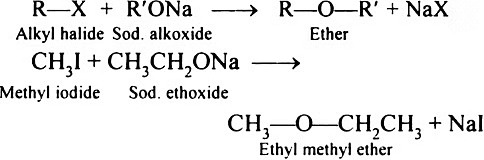
e.g. (i) n-PrOH
(ii) MeOH Me
(iii) t-BuOH t-Bu
t-Butyl ethyl ether
(This reaction produces a poor yield of ether because of the bulkiness of t-BuO-)
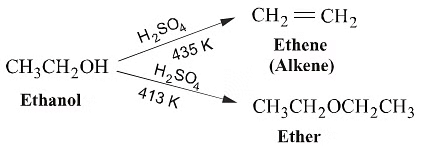
(2)Dehydration of Alcohol(Williamson's Continous Esterification Reaction )
When exposed to protic acids like sulfuric acid, alcohols undergo dehydration, resulting in the formation of alkenes and ethers under varying conditions.For instance, the dehydration of ethanol at 443 K in the presence of sulfuric acid produces ethene, while at 413 K, it yields ethoxyethane. This method is considered highly effective for obtaining products from primary alcohols.
General Reaction:
Mechanism:
Step-1:
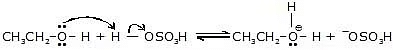
This is an acid-base reaction in which the alcohol accepts a proton from the sulfuric acid.
Step-2 :
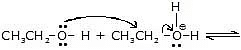
Another alcohol molecule acts as a nucleophile and attacks the protonated alcohol in a reaction.
Step-3 :
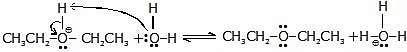
Another acid-base reaction converts the protonated ether to ether by transferring a proton to a molecule of water (or to another molecule of alcohol).
(3) From alkenes
(a) By the addition of alcohols in alkenes:
In the acid-catalyzed addition of alcohol to an alkene, the alkene is subjected to an excess of alcohol in the presence of an acid catalyst to generate an ether under optimal conditions. The hydrogen adds to the less substituted carbon, allowing the nucleophile to attack the more substituted carbon across the alkene, resulting in the formation of an ether.
General reaction:
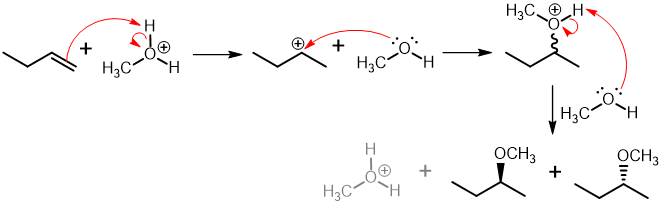
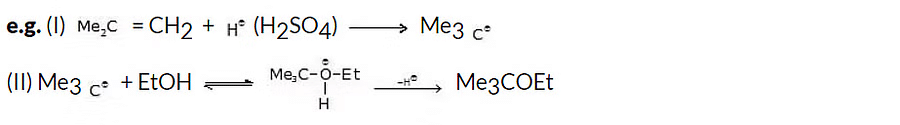
(b) Alkoxymercuration - demercuration:
- In the presence of mercuric acetate, an alkene interacts with alcohol, forming an alkoxymercury intermediate, which is later reduced with sodium borohydride to yield an ether.
- The acid-catalyzed addition of alcohols to alkenes involves treating an alkene with an excess of alcohol in the presence of an acid catalyst, resulting in the formation of an ether under appropriate conditions. For instance, passing 2-methylpropene and methanol over an acid catalyst produces 2-methoxy-2-methylpropane.
- Understanding the Alkoxymercuration-Demercuration Reduction mechanism can be challenging for organic chemistry students. The complexity doesn't lie in being more intricate than oxymercuration; rather, students often overlook the alcohol reagent and the alkyl group in the final product.
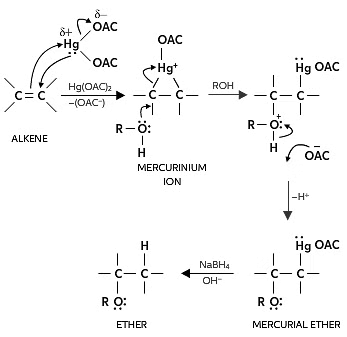
(i) RCH=CH2 +R'OH RCH(OR')CH3
(ii) CH2=CHCH3 +CH3CH(OH)CH 3
(iii) CH3CH=CHCH3 +CH3CH(OH)CH 2CH3
Chemical Reactions of Ethers
1. With HX
When ethers react with HX, alcohols, and alkyl halides are formed. The alcohol formed, reacts further with HX (which is in excess) to give alkyl halide. Hence, we get alkyl halide as the final product.
General reaction:
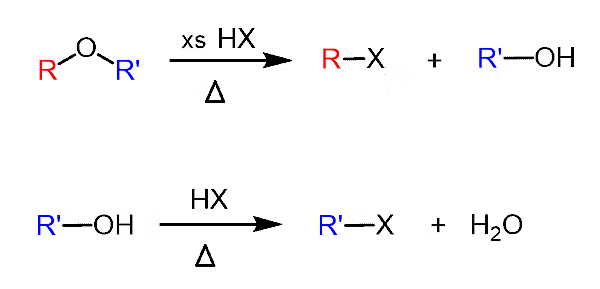
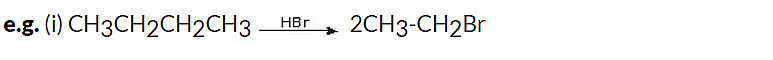
2. Reaction with Sulphuric Acid
Ethers dissolve in concentrated solutions of strong inorganic acids to form oxonium salts, i.e. ethers behave as Bronsted Lowry bases, and form alkyl hydrogen sulphate.
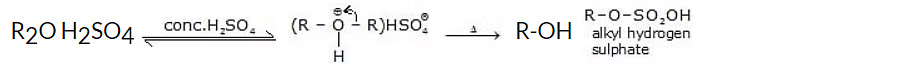
When heated with dilute H2SO4, ethers undergo hydrolysis to give alcohols.
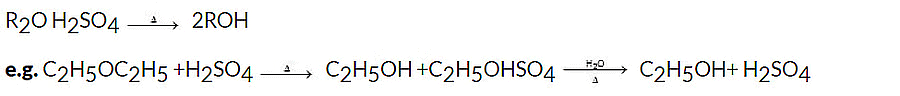
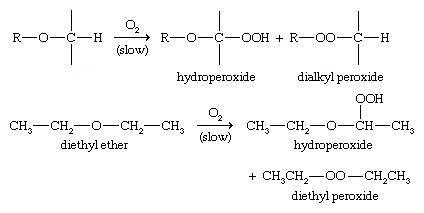
3. Autoxidation of ethers
When ethers are stored in the presence of atmospheric oxygen, they slowly oxidize to produce hydroperoxides and dialkyl peroxides, both of which are explosive. Such a spontaneous oxidation by atmospheric oxygen is called autoxidation.
General reaction:
4. Reaction with acid chlorides and anhydrides
Acid chlorides and acid anhydrides react with ethers in the presence of catalysts such as aluminum chloride or zinc chloride to form esters and haloalkanes.
Reagent : ZnCl2, AlCl3 etc.
General reaction: 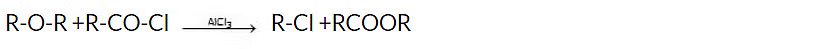
Mechanism:
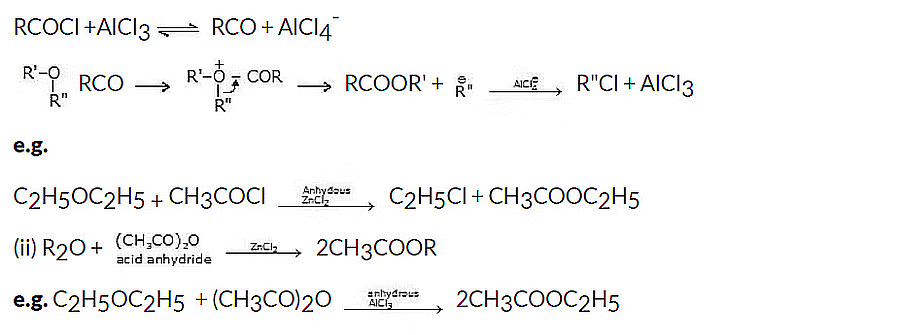
5. Reaction with carbon monoxide
Ethers react with CO at 125-180oC and a pressure of 500 atm, in the presence of BF3 plus a little water.

[Intext Questions]
6. Reaction with halogens
When treated with chlorine or Br, ether undergoes substitution, the extent of which depends on the conditions.

Mechanism
The reaction proceeds by a free-radical mechanism, and α-substitution occurs readily because of resonance stabilization of the intermediate radical.
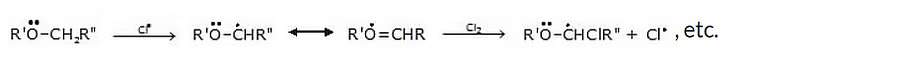
Uses of Ethers
Ethers find diverse applications across various industries. Here are some common uses:
1. Solvent: Ethers serve as effective solvents for a wide range of substances, including fats, oils, and various organic compounds. Diethyl ether, for instance, was historically used as a general anesthetic.
2. Laboratory Reagent: Ethers are frequently employed as reagents in organic synthesis and laboratory procedures. They play a crucial role in the creation of pharmaceuticals and other organic compounds.
3. Fuel Additive: Dimethyl ether, a type of ether, has been explored as a potential alternative fuel and fuel additive. It has been considered for use in diesel engines.
4. Extraction Solvent: Ethers are utilized in the extraction of natural products from plants. Their ability to dissolve a wide range of compounds makes them valuable in this context.
5. Polymer Industry: Some ethers are used as intermediates in the production of polymers. For example, ethyl methyl ether is used in the production of polyethylene.
6. Perfumes and Fragrances: Ethers contribute to the fragrance industry due to their pleasant-smelling nature. They are sometimes incorporated into perfumes and fragrances.
7. Paints and Coatings: Ethers can be used as solvents in the formulation of paints and coatings. They aid in achieving the desired consistency and application properties.
8. Preservative in Pharmaceuticals: Ethers, such as ethylene oxide, are used in the sterilization of medical equipment and as a preservative in certain pharmaceutical products.
Despite their usefulness, it's important to note that some ethers can be highly flammable and pose safety risks, so their handling and storage require careful consideration.
Solved Examples of Ethers
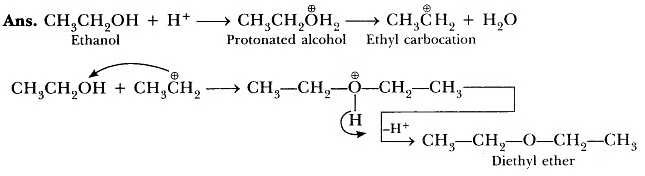
Question 1: 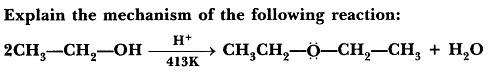
Question 2: Write the IUPAC name of the following
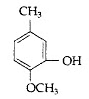 Answer: IUPAC name: 2-Methoxy-5-methyl phenol
Answer: IUPAC name: 2-Methoxy-5-methyl phenol
Question 3: Write the structure of the following compound: 2- Methyl-2ethoxypentane
Answer: 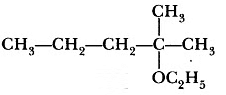
Question 4: The suitable reaction condition for the preparation of Methyl phenyl ether is
A. Benzene, MeBr
B. PhO-Na+, MeBr
C. Ph-Br, MeO-Na+
D. PhO-Na+, MeOH
Answer: B
Explanation:
Williamson's Synthesis
PhONa + MeBr-> PhOMe + NaBr
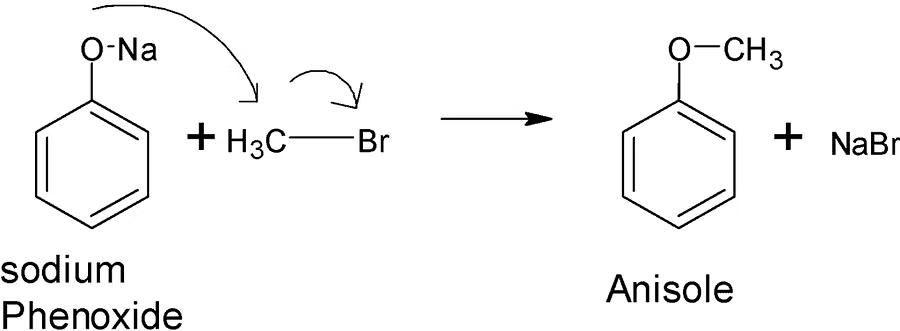
|
334 videos|656 docs|300 tests
|
FAQs on Ethers: Properties, Preparation and Reactions - Chemistry for JEE Main & Advanced
| 1. What are the physical properties of ethers? |  |
| 2. What are the methods of preparation of ethers? |  |
| 3. What are the chemical reactions of ethers? |  |
| 4. What are the uses of ethers? |  |
| 5. Can you provide a solved example of a reaction involving ethers? |  |















