JEE Advanced Previous Year Questions (2018 - 2024): Some Basic C oncepts of Chemistry | Chemistry for JEE Main & Advanced PDF Download
2024
No Question in JEE Advanced Exam from Chapter Some Basic Concepts of Chemistry in Year 2024 !
2023
Q1: H2S( 5 moles) reacts completely with acidified aqueous potassium permanganate solution. In this reaction, the number of moles of water produced is x, and the number of moles of electrons involved is y. The value of (x + y) is ________. [JEE Advanced 2023 Paper 2]
Ans: 18
We start with the balanced redox reaction :
2KMnO4 + 3H2SO4 + 5H2S → K2SO4 + 2MnSO4 + 5S + 8H2O
We want to find out the number of moles of water (x) produced and the number of moles of electrons (y) involved in this reaction.
From the balanced equation, we can see that 8 moles of water are produced from the reaction. So, we can say :
x = 8
Hydrogen sulfide (H2S) gets oxidized to sulfur (S) in this reaction. Each molecule of H2S loses 2 electrons during this process (as sulfur has an oxidation state of -2 in H2S and 0 in S).
So, for every mole of H2S, 2 moles of electrons are involved. And since 5 moles of H2S are reacting, the total number of moles of electrons involved is 5 × 2 = 10. So, we can say :
y = 10
We are asked to find the sum of x and y, so we add these two values together to get:
x + y = 8 + 10=18
Therefore, the value of (x + y) is 18.
Q2: The stoichiometric reaction of 516 g of dimethyldichlorosilane with water results in a tetrameric cyclic product X in 75% yield. The weight (in g) of X obtained is _______.
[Use, molar mass (g mol−1):H = 1, C = 12, O = 16, Si = 28, Cl = 35.5 ] [JEE Advanced 2023 Paper 1]
Ans: 222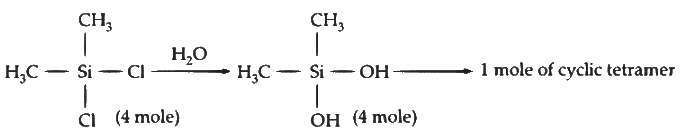
No. of moles = Given mass / Molar mass = 516 / 129 = 4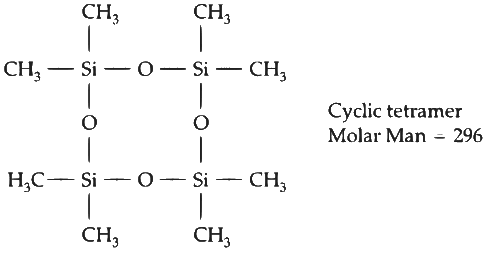
∴ Percentage yield = 75/100 = 0.75
∴ Mole formed of cyclic tetramer = 0.75
∴ Weight = 0.75 x 296 = 22g
2022
Q1: The treatment of an aqueous solution of 3.74 g of Cu(NO3)2 with excess KI results in a brown solution along with the formation of a precipitate. Passing H2S through this brown solution gives another precipitate X. The amount of X (in g ) is ___________.
[Given: Atomic mass of H = 1, N = 14, O = 16, S = 32, K = 39, Cu = 63, I = 127] [JEE Advanced 2022 Paper 1]
Ans: 0.31 to 0.33
Number of moles of Cu(NO3)2 = 3.74 / 187 = 0.02
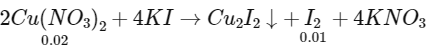
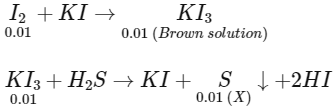
Number of moles of sulphur precipitated (X) = 0.01
Mass of sulphur precipitates (X) = 0.01 × 32 = 0.32 gm
Q2: To check the principle of multiple proportions, a series of pure binary compounds (PmQn) were analyzed and their composition is tabulated below. The correct option(s) is(are):
(a) If empirical formula of compound 3 is P3Q4, then the empirical formula of compound 2 is P3Q5.
(b) If empirical formula of compound 3 is P3Q2 and atomic weight of element P is 20 , then the atomic weight of Q is 45 .
(c) If empirical formula of compound 2 is PQ, then the empirical formula of the compound 1 is P5Q4.
(d) If atomic weight of P and Q are 70 and 35 , respectively, then the empirical formula of compound 1 is P2Q. [JEE Advanced 2022 Paper 2]
Ans: (b) & (c)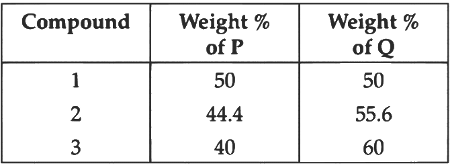
(A) If emperical formula of compound 3 is P3Q4 then its molar ratio will be 40/3: 60/4
=40 / 3 × 4 / 60 = 16 / 18 = 0.88
If empirical formula of compound 2 is P3Q5, then its molar ratio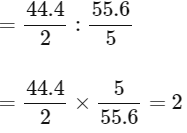
Since molar ratio of both the compound is not equal
So, option (A) is not correct.
(B) If empirical formula of compound 3 is P3Q2, i.e.,
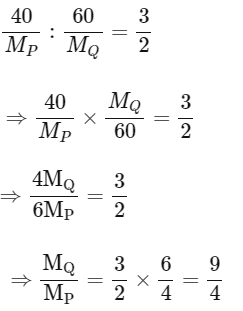
and MP = 20 (given)
So, MQ / 20 = 9/4
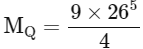
MQ = 45
So, option (B) is correct.
(C) If empirical formula of compound 2 is PQ, So the molar ratio is
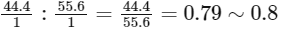
Empirical formula of compound 1 is P5Q4
so the molar ratio is 50/5 : 50/4.
= 50 / 5 × 4 / 50 = 4 / 5 = 0.8
Since, molar ratio of both the compound is equal hence, state (C) is correct.
So, option (C) is correct.
(D) MP = 70, MQ = 35
Molar ratio of compound 1 is
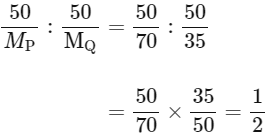
Hence, empirical formula of compound PQ2.
So, option (D) is incorrect.
2020
Q1: In the chemical reaction between stoichiometric quantities of KMnO4 and KI in weakly basic solution, what is the number of moles of I2 released for 4 moles of KMnO4 consumed? [JEE Advanced 2020 Paper 2]
Ans: 6
In alkaline medium : Iodide is oxidised to iodate
2MnO4− + H2O + I− ⟶ 2MnO2 + 2OH− + IO3−
But in weakly basic solution :
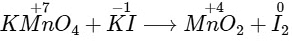 Eq. of KMnO4 = Eq. of I2
Eq. of KMnO4 = Eq. of I24 x 3 = n x 2
⇒ n = 6
Q2: 5.00 mL of 0.10 M oxalic acid solution taken in a conical flask is titrated against NaOH from a burette using phenolphthalein indicator. The volume of NaOH required for the appearance of permanent faint pink color is tabulated below for five experiments. What is the concentration, in molarity, of the NaOH solution? [JEE Advanced 2020 Paper 1]
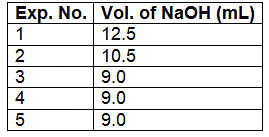 Ans: 0.11
Ans: 0.11Oxalic acid solution titrated with NaOH solution using phenolphthalein as an indicator.

Equivalent of H2C2O4 reacted = Equivalent of NaOH reacted
=
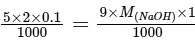
M(NaOH) = 1/9
= 0.11
 |
Download the notes
JEE Advanced Previous Year Questions (2018 - 2024): Some Basic C oncepts of Chemistry
|
Download as PDF |
2019
Q1: The mole fraction of urea in an aqueous urea solution containing 900 g of water is 0.05. If the density of the solution is 1.2 g cm−3, then molarity of urea solution is ................
(Given data : Molar masses of urea and water are 60 g mol−1 and 18 g mol−1, respectively)
[JEE Advanced 2019 Paper 2]
Ans: 2.98
Molarity
(M) = Number of moles of solute x 1000 / Volume of solution (in mL)
Also, volume = Mass / Density
Given, mole fraction of urea (Xurea) = 0.05
Mass of water = 900 g
Density = 1.2 g/cm3
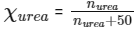 [∵ Moles of water = 900/18 = 50]
[∵ Moles of water = 900/18 = 50]
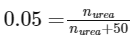

= 881.57 mL
Now, molarity
Number of moles of solute x 1000 / Volume of solution (in mL)
= 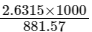
= 2.98 M
Q2: The amount of water produced (in g) in the oxidation of 1 mole of rhombic sulphur by conc. HNO3 to a compound with the highest oxidation state of sulphur is ..............
(Given data : Molar mass of water = 18 g mol−1) [JEE Advanced 2019 Paper 2]
Ans: 288
When rhombic sulphur (S8) is oxidised by conc. HNO3 then H2SO4 is obtained and NO2 gas is released.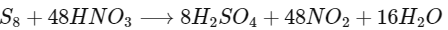
1 mole of rhombic sulphur produces = 16 moles of H2O
∴ Mass of water = 16 × 18 (molar mass of H2O) = 288 g
2018
Q1: To measure the quantity of MnCl2 dissolved in an aqueous solution, it was completely converted to KMnO4 using the reaction,
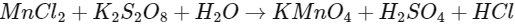 (equation not balanced).
(equation not balanced).
Few drops of concentrated HCl were added to this solution and gently warmed. Further, oxalic acid (225 mg) was added in portions till the colour of the permanganate ion disappeared. The quantity of MnCl2 (in mg) present in the initial solution is ____________.
(Atomic weights in g mol−1: Mn = 55, Cl = 35.5 ) [JEE Advanced 2018 Paper 2]
Ans: 126
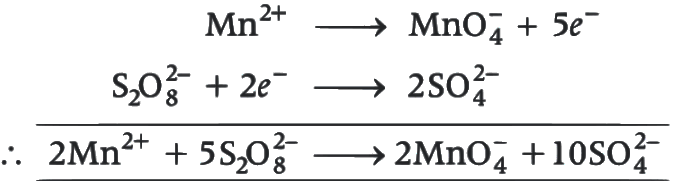
Also,
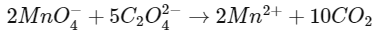
Hence, 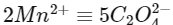
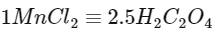
Oxalic acid taken = 225 mg
= 225 / 90
= 2.5 millimoles
Hence, MnCl2 = 1 millimole
= (55 + 71)
= 126 mg
|
353 videos|587 docs|309 tests
|
FAQs on JEE Advanced Previous Year Questions (2018 - 2024): Some Basic C oncepts of Chemistry - Chemistry for JEE Main & Advanced
| 1. What are some basic concepts of chemistry covered in the JEE Advanced exam? |  |
| 2. How can I prepare for the basic concepts of chemistry for the JEE Advanced exam? |  |
| 3. What are some important topics related to the basic concepts of chemistry that I should focus on for the JEE Advanced exam? |  |
| 4. Are there any common misconceptions or traps to be aware of while studying the basic concepts of chemistry for the JEE Advanced exam? |  |
| 5. Can you suggest some online resources or study materials for the basic concepts of chemistry for the JEE Advanced exam? |  |