Class 7 Exam > Class 7 Notes > Year 7 Chemistry (Cambridge) > Acidity and indicators
Acidity and indicators | Year 7 Chemistry (Cambridge) - Class 7 PDF Download
Introduction to Acids and Alkalis
- Acids and alkalis are common substances with distinct properties.
- Acids: Examples include hydrochloric acid (found in stomachs), sulfuric acid (in car batteries), and citric acid (in citrus fruits like lemons).
- Alkalis: Examples include sodium hydroxide (in cleaning products and soaps) and potassium hydroxide (in soaps).
Properties of Acids and Alkalis
- Acids: Sour taste; can be corrosive or irritant. Examples: vinegar, fizzy drinks.
- Alkalis: Soapy feel; can be corrosive or irritant. Examples: soap, bleach.
Safety with Acids and Alkalis
- Some acids and alkalis are safer (e.g., vinegar, soaps) while others are more dangerous (e.g., car battery acids, cleaning products).
- Protective equipment like gloves and goggles may be required when handling stronger acids and alkalis.
Chemical Symbols and Composition
- Acids: Typically start with hydrogen in their chemical formulas (e.g., HCl for hydrochloric acid, H2SO4 for sulfuric acid).
- Alkalis: Often end with hydroxide in their formulas (e.g., NaOH for sodium hydroxide).
Question for Acidity and indicatorsTry yourself: Which of the following acids is commonly found in citrus fruits like lemons?View Solution
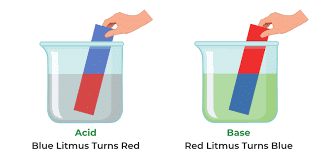
Using Indicators to Identify Acids and Alkalis
- Colorless solutions of acids and alkalis can be identified using universal indicator.
- Acids: Turn universal indicator colors like red, orange, or yellow (pH range 0-6).
- Alkalis: Turn universal indicator colors like green, blue, or purple (pH range 8-14).
- pH 7 is neutral, neither acidic nor alkaline.
Strength of Acids and Alkalis
- pH scale ranges from 0 (strong acids) to 14 (strong alkalis).
- Weak Acids: Examples include tea and coffee (pH close to neutral).
- Strong Acids: Examples include vinegar (pH around 2) and stomach acid.
- Weak Alkalis: Everyday items like toothpaste and soap (pH close to neutral).
- Strong Alkalis: Examples like bleach (pH higher, towards 14).
Conclusion
- Understanding acids and alkalis helps in identifying their properties and uses in everyday life.
- The pH scale and universal indicator are crucial tools in distinguishing between acids and alkalis based on their strength and characteristics.
The document Acidity and indicators | Year 7 Chemistry (Cambridge) - Class 7 is a part of the Class 7 Course Year 7 Chemistry (Cambridge).
All you need of Class 7 at this link: Class 7
|
7 videos|15 docs|8 tests
|
FAQs on Acidity and indicators - Year 7 Chemistry (Cambridge) - Class 7
| 1. What are some common indicators used to determine the acidity or alkalinity of a substance? |  |
Ans. Some common indicators used to determine the acidity or alkalinity of a substance include litmus paper (which turns red in acidic solutions and blue in alkaline solutions), phenolphthalein (which turns pink in alkaline solutions), and methyl orange (which changes color from red to yellow in acidic solutions).
| 2. How does the pH scale measure the acidity or alkalinity of a substance? |  |
Ans. The pH scale measures the acidity or alkalinity of a substance on a scale of 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most alkaline. Substances with a pH below 7 are considered acidic, while substances with a pH above 7 are considered alkaline.
| 3. What are some common household items that are acidic or alkaline? |  |
Ans. Some common household items that are acidic include vinegar, lemon juice, and soda, while items that are alkaline include baking soda, soap, and bleach.
| 4. How do acids and alkalis react with each other? |  |
Ans. Acids and alkalis react with each other in a neutralization reaction, where they combine to form water and a salt. During this reaction, the acidic properties of the acid and the alkaline properties of the alkali are neutralized.
| 5. How can you determine if a substance is an acid or an alkali without using indicators? |  |
Ans. One way to determine if a substance is an acid or an alkali without using indicators is to taste it (with caution). Acids taste sour, while alkalis taste bitter. However, it is not recommended to taste substances to determine their acidity or alkalinity due to potential health risks.
Related Searches





















