JEE Exam > JEE Notes > Chemistry for JEE Main & Advanced > Mind Map: Chemical Kinetics
Mind Map: Chemical Kinetics | Chemistry for JEE Main & Advanced PDF Download
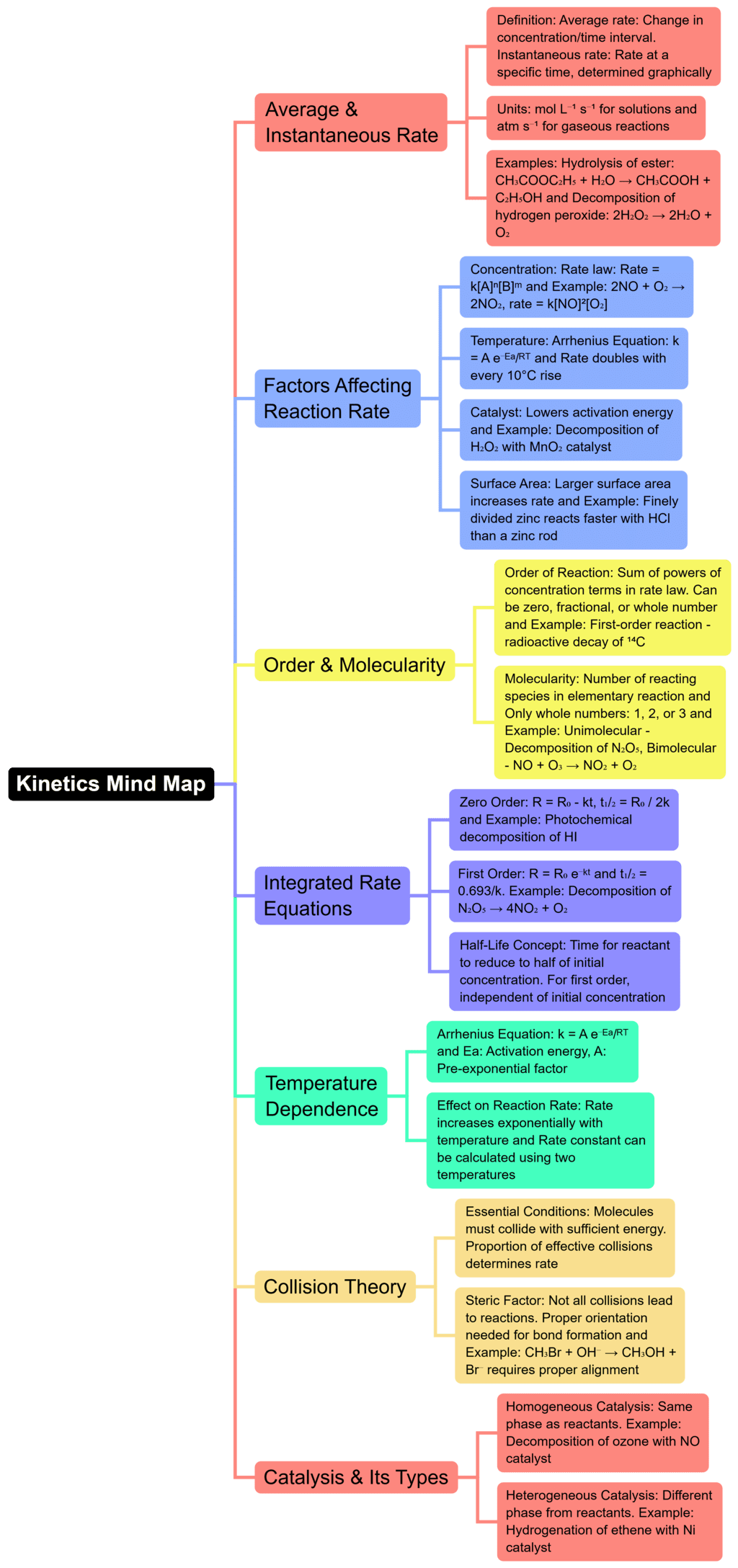
The document Mind Map: Chemical Kinetics | Chemistry for JEE Main & Advanced is a part of the JEE Course Chemistry for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
334 videos|651 docs|300 tests
|
FAQs on Mind Map: Chemical Kinetics - Chemistry for JEE Main & Advanced
| 1. What is the significance of the rate law in chemical kinetics? |  |
Ans. The rate law expresses the relationship between the rate of a chemical reaction and the concentration of its reactants. It is significant because it helps in understanding how different factors affect the speed of a reaction. By determining the rate law, one can predict how changes in concentration influence the rate, which is crucial for both theoretical studies and practical applications, such as in industrial processes.
| 2. How do temperature and catalysts affect reaction rates? |  |
Ans. Temperature increases the kinetic energy of molecules, leading to more frequent and effective collisions, thus generally increasing reaction rates. Catalysts lower the activation energy required for a reaction, allowing it to proceed more quickly without being consumed in the process. Both factors are essential in optimizing reactions in both laboratory and industrial settings.
| 3. What are the different types of reaction mechanisms, and how do they relate to chemical kinetics? |  |
Ans. Reaction mechanisms describe the step-by-step sequence of elementary reactions that lead to the overall reaction. They can be classified as unimolecular, bimolecular, or termolecular, depending on the number of reactant molecules involved in each step. Understanding these mechanisms helps chemists to derive the rate laws and predict the behavior of reactions under various conditions.
| 4. How can the half-life of a reaction be determined, and what does it indicate about the reaction order? |  |
Ans. The half-life of a reaction is the time required for the concentration of a reactant to decrease to half of its initial value. It varies depending on the order of the reaction. For first-order reactions, the half-life is constant, while for second-order reactions, it depends on the initial concentration. Analyzing half-lives helps in determining the order of a reaction and its kinetics.
| 5. What role does the Arrhenius equation play in chemical kinetics? |  |
Ans. The Arrhenius equation relates the rate constant of a reaction to the temperature and activation energy. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin. This equation is crucial for understanding how temperature influences reaction rates and for calculating activation energies experimentally.
Related Searches
















