JEE Exam > JEE Notes > Chemistry for JEE Main & Advanced > Mind Map: Chemical Bonding and Molecular Structure
Mind Map: Chemical Bonding and Molecular Structure | Chemistry for JEE Main & Advanced PDF Download
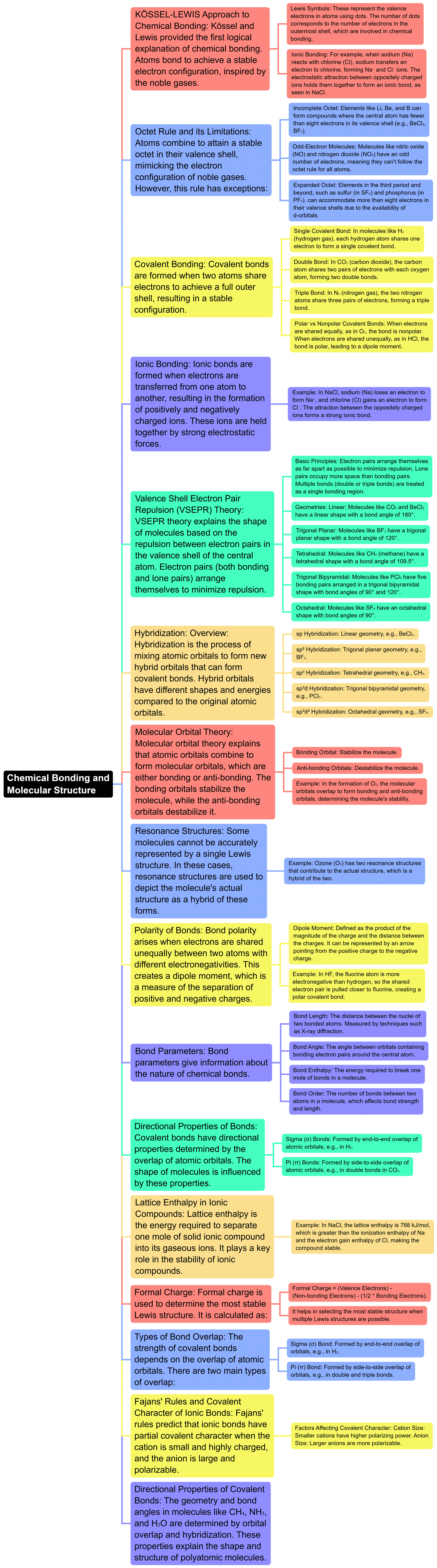
The document Mind Map: Chemical Bonding and Molecular Structure | Chemistry for JEE Main & Advanced is a part of the JEE Course Chemistry for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
334 videos|656 docs|300 tests
|
FAQs on Mind Map: Chemical Bonding and Molecular Structure - Chemistry for JEE Main & Advanced
| 1. What are the main types of chemical bonds and how do they differ from each other? |  |
Ans. The main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. Ionic bonds occur when electrons are transferred from one atom to another, resulting in the formation of charged ions (e.g., Na⁺ and Cl⁻ in NaCl). Covalent bonds involve the sharing of electron pairs between atoms (e.g., in H₂O, each hydrogen atom shares an electron with oxygen). Metallic bonds are characterized by a sea of delocalized electrons that allow for conductivity and malleability, typically found in metals.
| 2. How does the concept of electronegativity influence chemical bonding? |  |
Ans. Electronegativity is the tendency of an atom to attract electrons in a chemical bond. It plays a crucial role in determining the type of bond formed between two atoms. If the difference in electronegativity between the two atoms is large (typically greater than 1.7), an ionic bond is likely to form. If the difference is moderate (between 0.4 and 1.7), a polar covalent bond is formed, where the electrons are shared unequally. If the difference is small (less than 0.4), a nonpolar covalent bond occurs, with electrons shared equally.
| 3. What is the significance of molecular geometry in understanding chemical bonding? |  |
Ans. Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule, which significantly affects its physical and chemical properties. The shape of a molecule influences its reactivity, polarity, phase of matter, color, magnetism, biological activity, and more. Understanding molecular geometry helps predict how molecules will interact during chemical reactions and can be determined using theories such as VSEPR (Valence Shell Electron Pair Repulsion) theory.
| 4. Can you explain hybridization and its role in molecular structure? |  |
Ans. Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate the bonding of atoms in a molecule. This process explains the geometry of molecular structures. For example, in methane (CH₄), the carbon atom undergoes sp³ hybridization, resulting in four equivalent hybrid orbitals that arrange themselves tetrahedrally to minimize repulsion. Hybridization helps in understanding the type of bonds (single, double, or triple) and the angles between them in a molecule.
| 5. How do resonance structures contribute to the stability of molecules? |  |
Ans. Resonance structures are different Lewis structures that represent the same molecule, differing only in the placement of electrons. These structures illustrate that the actual molecule is a hybrid of these forms, leading to a more stable configuration than any single resonance structure. For example, in benzene (C₆H₆), the resonance between two structures helps delocalize electrons across the ring, contributing to its stability and unique chemical properties. This delocalization lowers the overall energy of the molecule.
Related Searches
















