UPSC Mains Answer PYQ 2024: Geology Paper 2 (Section- A) | Geology Optional for UPSC PDF Download
SECTION A
Q1: Answer the following questions in about 150 words each: (10 × 5 = 50 Marks)
(a) What are the different types of rotational axes of symmetry present in a crystal? What are the different types of twinning observed in quartz?
Ans: Crystals exhibit symmetry, which is crucial for understanding their physical properties. The rotational axes of symmetry define the ways in which a crystal can be rotated to coincide with itself, while twinning in crystals refers to the intergrowth of two or more crystals in a symmetrical manner.
Types of Rotational Axes of Symmetry:
1-fold axis: No symmetry (no rotation).
2-fold axis: A crystal can be rotated 180° to coincide with its original position (e.g., calcite).
3-fold axis: A crystal can be rotated 120° to coincide (e.g., borax).
4-fold axis: A crystal can be rotated 90° to coincide (e.g., fluorite).
6-fold axis: A crystal can be rotated 60° to coincide (e.g., graphite).
Types of Twinning in Quartz:
- Contact Twinning: Occurs when two crystals share a face or edge, with a specific symmetry.
- Penetration Twinning: Occurs when two crystals intergrow by intersecting at an angle.
- Multiple Twinning: Occurs when more than two crystal parts join in a complex pattern.
Example: Quartz commonly exhibits contact twinning, leading to complex shapes and optical effects in certain mineral forms like amethyst.
Rotational axes help define the symmetry of crystals, while twinning leads to the formation of unique crystal intergrowths, as seen in minerals like quartz.
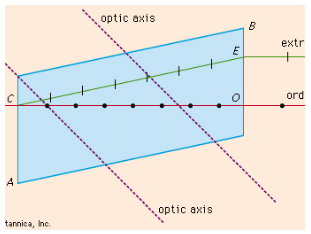
(b) How does one define "Double refraction" and "Birefringence" of an anisotropic mineral? Write with the help of suitable sketches.
Ans: Anisotropic minerals are minerals that exhibit different optical properties in different crystallographic directions. Two important optical properties of anisotropic minerals are double refraction and birefringence.
Double Refraction and Birefringence in Anisotropic Minerals
1. Double Refraction
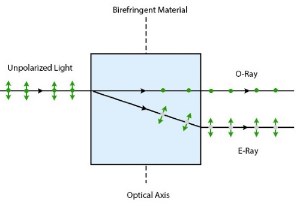
- Double refraction, also known as birefringence, is a phenomenon in anisotropic minerals where a single ray of unpolarized light splits into two separate rays upon entering the mineral.
- Explanation:
- The splitting occurs due to the mineral's internal structure, where the speed of light varies along different crystallographic directions.
- The two rays are:
- Ordinary Ray (O-ray): Travels with a constant velocity irrespective of direction.
- Extraordinary Ray (E-ray): Travels with varying velocity depending on its path through the crystal.
- Examples: Calcite is a classic example of a mineral exhibiting strong double refraction.
2. Birefringence
- Birefringence is the numerical difference between the refractive indices of the ordinary and extraordinary rays in an anisotropic mineral.
- Formula:
- Δn=ne−no
- Where ne is the refractive index of the extraordinary ray and non_ono is that of the ordinary ray.
- Explanation:
- It quantifies the optical anisotropy of the mineral.
- High birefringence leads to strong interference colors observed under a polarizing microscope.
- Practical Use:
- Birefringence is critical in identifying minerals using optical techniques in thin sections.
Key Points in Optical Mineralogy:
- Anisotropy: Minerals with anisotropic properties (non-cubic systems) show birefringence and double refraction.
- Optical Indicatrix: The geometrical representation of light velocities in a mineral aids in understanding birefringence.
- Applications:
- Used in mineral identification.
- Helps determine optical properties like extinction angle and optic sign (positive or negative).
Double refraction and birefringence are important optical properties of anisotropic minerals that result from the different refractive indices in different crystallographic directions. These properties are key in identifying and studying anisotropic minerals in geology and mineralogy.
(c) Show diagrammatically the characteristics of binary eutectic system under 1 atmosphere (1 atm pressure). How does one explain the formation of porphyritic basic rock with phenocryst of plagioclase in a groundmass with plagioclase and clinopyroxene with the help of a suitable binary eutectic system?
Ans:
- A binary eutectic system consists of two components that form a eutectic mixture at a specific composition and temperature.
- The characteristics of a binary eutectic system under 1 atm pressure can be represented diagrammatically.
Characteristics of Binary Eutectic System Under 1 Atmosphere
Key Features:
- A binary eutectic system involves two components that can coexist as liquid and solid at a specific eutectic composition and temperature under 1 atm pressure.
- Phases: Includes liquid, solid A, and solid B.
- Eutectic Point: The lowest temperature at which the liquid phase can exist; both components crystallize simultaneously in a fixed ratio.
Diagram Explanation:
- The phase diagram consists of:
- Liquidus Curve: Separates the liquid phase from the two-phase liquid + solid region.
- Solidus Curve: Indicates temperatures below which only solid phases exist.
- Eutectic Point: Intersection of solidus and liquidus lines where the two solids crystallize simultaneously.
Simplified Diagram:
Formation of Porphyritic Basic Rock with Plagioclase Phenocrysts
Explanation with Binary Eutectic System:
- System: Consider the diopside-anorthite system as a representative binary eutectic system.
- Initial Stage:
- The magma initially cools slowly at depth, leading to the formation of large plagioclase phenocrysts (early crystallization due to supercooling).
- Plagioclase forms due to the liquidus curve's inclination toward the plagioclase composition.
- Subsequent Cooling:
- As the magma cools further, it reaches the eutectic point.
- Both plagioclase and clinopyroxene crystallize together from the remaining liquid.
- Final Rock Texture: The phenocrysts of plagioclase (formed earlier) are embedded in a fine-grained groundmass composed of plagioclase and clinopyroxene, reflecting the eutectic crystallization.
Relevance of Binary Eutectic System:
- The eutectic system explains the simultaneous crystallization of plagioclase and clinopyroxene in the groundmass.
- The earlier nucleation of plagioclase corresponds to the solidus-liquidus path of a plagioclase-rich composition.
Diagram:
- The characteristics of a binary eutectic system under 1 atm pressure can be represented diagrammatically.
- The formation of porphyritic basic rock with phenocrysts of plagioclase can be explained using a binary eutectic system with plagioclase and clinopyroxene.
(d) Explain the processes involved in magmatic differentiation.
Ans: Magmatic differentiation refers to the process by which different types of rocks and minerals are formed from a single magma as it cools. This process explains the variety of igneous rocks found in the Earth's crust and is fundamental in understanding the evolution of magmatic systems.
Processes Involved in Magmatic Differentiation
Crystallization:
As magma cools, different minerals crystallize at different temperatures. For example, olivine crystallizes first, followed by pyroxenes, feldspars, and quartz.
Bowen's Reaction Series describes the sequence of mineral crystallization from magma, where high-temperature minerals (like olivine and pyroxene) crystallize first, while low-temperature minerals (like quartz and feldspar) crystallize later.
Partial Melting:
Not all minerals melt at the same temperature. As magma undergoes partial melting, certain minerals melt first, enriching the melt in specific elements, such as silica, potassium, or sodium.
Example: In granite formation, felsic minerals like quartz and feldspar melt first, while mafic minerals remain solid, leading to the formation of a more silicic magma.
Fractional Crystallization:
This process occurs when the crystals formed from cooling magma are separated from the remaining liquid. These crystals may sink to the bottom or remain suspended, thereby enriching the liquid in certain elements.
Example: When basaltic magma cools, early-formed olivine and pyroxene sink, and the remaining magma becomes more enriched in silica, leading to the formation of andesite or dacite.
Magma Mixing:
Different magmas from various sources may mix during cooling, creating rocks with characteristics of both parent magmas.
Example: Mixing of mafic and felsic magmas can lead to the formation of intermediate rocks like andesite.
Assimilation:
The process by which magma incorporates material from surrounding rocks (country rocks), leading to changes in its composition.
Example: Magma may assimilate limestone from its surroundings, enriching the melt in calcium and forming calc-alkaline rocks.
Magmatic differentiation is a complex process involving crystallization, partial melting, fractional crystallization, magma mixing, and assimilation. These processes explain the diversity of igneous rocks and the chemical variation within Earth's crust.
(e) With the help of suitable diagrams, describe Folk's graphic classification of carbonate rocks.
Ans: Folk's graphic classification of carbonate rocks is a widely used method to categorize and describe different types of carbonate rocks based on their texture and composition. This classification system was developed by Robert L. Folk in 1959 and has since been used by geologists to better understand the characteristics of carbonate rocks.
Folk’s Graphic Classification of Carbonate Rocks
Folk’s classification system is a widely used method to categorize carbonate rocks based on textural components and depositional characteristics. Below is a structured explanation with diagrams:
Components of Folk's Classification
- Allochemical Components (Allochems):
- Grains formed and transported before being deposited.
- Types:
- Skeletal grains: Fossil fragments.
- Ooids: Spherical coated grains.
- Peloids: Micritic grains without internal structure.
- Intraclasts: Fragmented carbonate sediments.
- Orthochemical Components:
- Material formed in situ after deposition.
- Types:
- Micrite: Microcrystalline calcite forming the matrix.
- Sparite: Sparry calcite acting as cement.
Textural Classification
- Rocks are classified based on the proportion of allochems, micrite, and sparite.
- Allochemical Rocks:
- Dominated by allochems.
- Types:
- Oosparite: Cemented ooids with sparite.
- Biomicrite: Skeletal grains in micrite.
- Intramicrite: Intraclasts with micrite matrix.
- Orthochemical Rocks:
- Dominated by micrite and sparite.
- Types:
- Micrite: Composed of microcrystalline calcite.
- Sparite: Comprised of sparry calcite.
Classification Framework
- Based on the proportion of micrite (matrix) and sparite (cement):
- Micritic Rocks: Dominated by micrite.
- Sparitic Rocks: Dominated by sparite.
- Grain-supported Rocks: Contain allochems supported by grains, with minimal micrite.
Diagram Representation
Folk's graphic classification of carbonate rocks provides a systematic way to categorize and describe the various types of carbonate rocks based on their texture and composition. By using this classification system, geologists can better understand the characteristics and origins of carbonate rocks, leading to improved interpretations of sedimentary environments and geological history.
Q2:
(a) What are the symmetry elements present in the normal class of an isometric system? Write the Hermann-Mauguin notation of the normal class of isometric system. Plot the face (hkl) and deduce the form generated by operation of symmetry elements from the face (hkl) on a stereogram of the normal class of isometric system. (15 Marks)
Ans: The isometric (or cubic) crystal system is one of the seven crystal systems, where the unit cell is characterized by three axes of equal length and perpendicular to each other. The symmetry elements of the isometric system dictate the behavior of crystal faces, and these elements play a key role in determining the overall symmetry of the crystal.
Symmetry Elements in the Isometric System
- Three 4-fold rotational axes: These axes pass through the center of the crystal and divide the crystal into four equivalent parts.
- Three 2-fold rotational axes: These axes divide the crystal into two equivalent parts.
- Six mirror planes: These reflect the crystal along its axes, creating a symmetrical counterpart.
- Four 3-fold rotational axes: These axes divide the crystal into three equal parts and rotate them symmetrically.
- Inversion center: The inversion center is a point from which symmetry is achieved in all directions.
- Identity operation (1-fold axis): The simplest symmetry operation that represents no change.
Hermann-Mauguin Notation of the Normal Class of Isometric System
- The notation for the normal class of the isometric system is denoted as m3m, which describes:
- m: Mirror plane symmetry.
- 3: 3-fold rotational symmetry.
- m: Another mirror plane perpendicular to the first.
Plotting the Face (hkl) and Symmetry Operations
- Face (hkl) represents the orientation of the crystal face relative to the unit cell axes.
- By applying the symmetry elements (e.g., rotation and mirror operations) to the (hkl) face, a form is generated where the faces are symmetrically distributed around the crystal.
Stereogram: A stereogram of the isometric system shows how the symmetry elements act upon the faces. For instance, after rotating the (hkl) face by 90° (using the 4-fold axis), we get identical faces in different orientations, generating the characteristic cubic symmetry.
The isometric crystal system exhibits a high degree of symmetry, and its Hermann-Mauguin notation m3m represents the symmetry elements that influence the arrangement of faces within the crystal.
(b) Draw and describe the structure of mica group of minerals. Describe the chemical composition and optical properties of minerals of mica group. (15 Marks)
Ans: The mica group consists of sheet silicate minerals known for their perfect cleavage in one direction. These minerals are found widely in igneous and metamorphic rocks and are essential for understanding rock textures and mineralogy.
Structure of Mica Group Minerals
- Sheet-like Structure: Mica minerals have a two-dimensional layered structure, where each layer consists of silicate tetrahedra bonded together with oxygen atoms.
- The tetrahedra share common oxygen atoms, forming a strong hexagonal sheet.
- Interlayer cations (e.g., potassium, magnesium, or iron) are situated between the layers, contributing to the cleavage along the layers.
Chemical Composition
- The general formula for mica minerals is KAl2(Si3Al)O10(OH)2 for muscovite, and K(Mg,Fe)3(AlSi3O10)(OH)2 for biotite.
- Muscovite: Composed primarily of potassium, aluminum, silicon, oxygen, and hydroxyl groups.
- Biotite: Composed of potassium, iron, magnesium, aluminum, and silicon.
Optical Properties
- Transparency: Mica minerals are typically transparent or translucent, allowing light to pass through their thin layers.
- Color: Muscovite is generally colorless to light brown, while biotite is dark brown or black due to the presence of iron and magnesium.
- Cleavage: Micas exhibit perfect cleavage in one direction, which allows them to be split into thin, flexible sheets.
- Refractive Index: Mica minerals have a moderate refractive index, usually between 1.5 and 1.6.
- Birefringence: Micas typically show low birefringence due to the near isotropy of their individual layers.
- Pleochroism: Biotite shows strong pleochroism, meaning it changes color when viewed at different angles.
Example
- Muscovite: Commonly found in granite and schist.
- Biotite: Found in basalt and other igneous rocks.
The mica group consists of minerals with a unique sheet structure, perfect cleavage, and distinctive optical properties. These minerals are integral to understanding rock formation and texture.
(c) Define polymorphism and discuss different types of polymorphic transitions. What are the different types of polymorphs of SiO2 and Al2SiO5? (20 Marks)
Ans: Polymorphism refers to the ability of a substance to exist in more than one structural form. This phenomenon is common in minerals and occurs when the same chemical composition can crystallize in different ways, depending on temperature and pressure conditions.
Types of Polymorphic Transitions
- High-Temperature Polymorphs: Formed at elevated temperatures, these polymorphs are stable at high temperatures and transform to other forms as the temperature decreases.
- Low-Temperature Polymorphs: These are stable at lower temperatures and may transform into high-temperature polymorphs upon heating.
- Pressure-Induced Polymorphs: These transitions occur due to changes in pressure, such as in deep Earth environments.
- Reversible Polymorphic Transitions: Some polymorphs can reversibly change from one form to another depending on temperature or pressure conditions.
- Irreversible Polymorphic Transitions: Other polymorphs do not revert back after undergoing transformation.
Polymorphs of SiO2 (Silicon Dioxide)
- Quartz: The most stable and common polymorph of SiO2 at surface conditions. It forms hexagonal crystals.
- Tridymite: High-temperature polymorph of SiO2, stable at temperatures above 870°C.
- Cristobalite: Another high-temperature form of SiO2, stable at temperatures above 1470°C.
- Coesite: A high-pressure polymorph formed at extreme conditions (e.g., during meteorite impacts).
- Stishovite: A high-pressure polymorph formed under conditions of very high pressure.
Polymorphs of Al2SiO5 (Aluminum Silicate)
- Andalusite: Forms at moderate temperatures and pressures.
- Kyanite: Stable at high pressure and low temperature, often found in metamorphic rocks.
- Sillimanite: Forms at high temperatures and pressures and is stable at high-grade metamorphic conditions.
Example: Quartz and Cristobalite both have the same chemical composition (SiO2) but crystallize in different forms depending on temperature.
Polymorphism in minerals like SiO2 and Al2SiO5 illustrates the adaptability of substances to different environmental conditions, affecting their stability and properties. Understanding polymorphism is essential for mineralogy, petrology, and geophysics.
Q3:
(a) Describe the mineral reactions in prograde metamorphism of argillaceous sedimentary rocks with appropriate diagrams. (15 Marks)
Ans: Prograde metamorphism is the process by which sedimentary rocks undergo changes in mineral composition and texture due to increased temperature and pressure. In the case of argillaceous sedimentary rocks, which are rich in clay minerals, the mineral reactions that occur during prograde metamorphism are crucial in determining the final metamorphic rock that is formed.
Prograde Metamorphism of Argillaceous Sedimentary Rocks
Prograde metamorphism refers to the mineralogical and textural transformations in rocks subjected to increasing temperature and pressure conditions. For argillaceous (clay-rich) sedimentary rocks, these changes progress through a well-defined sequence of mineral assemblages.
Key Mineral Reactions in Prograde Metamorphism
- Low-Grade Metamorphism
- Minerals Formed: Chlorite, muscovite, and sericite.
- Processes:
- Dehydration of clay minerals like kaolinite and illite.
- Formation of fine-grained phyllosilicates such as chlorite and muscovite.
- Reaction: Kaolinite+Quartz→Muscovite+Water
- Rock Type: Slate or phyllite.
- Medium-Grade Metamorphism
- Minerals Formed: Biotite, garnet, and staurolite.
- Processes:
- Further dehydration reactions.
- Formation of biotite from muscovite and chlorite.
- Growth of garnet and staurolite as index minerals.
- Reaction: Chlorite+Quartz→Garnet+Water
- Rock Type: Schist.
- High-Grade Metamorphism
- Minerals Formed: Sillimanite, kyanite, and feldspars.
- Processes:
- Transition to high-temperature minerals such as sillimanite from kyanite.
- Breakdown of muscovite to form feldspar.
- Reaction: Kyanite→Sillimanite
- Rock Type: Gneiss.
Diagrams
- P-T Diagram:
- Illustrates the stability fields of key minerals (e.g., chlorite, biotite, garnet, kyanite, and sillimanite).
- Shows the progressive path of increasing pressure and temperature
- AFM Diagram:
- Represents the chemical evolution of pelitic rocks.
- Tracks the changes in mineral assemblages during progressive metamorphism.
The mineral reactions that occur during prograde metamorphism of argillaceous sedimentary rocks play a crucial role in the transformation of these rocks into metamorphic rocks. Through processes such as dehydration, recrystallization, and metamorphic reactions, the original mineral composition of the sedimentary rocks is altered to form new mineral assemblages that are characteristic of metamorphic rocks.
(b) Write the mineralogy and texture of basalt. How does basaltic magma form in deep earth? (15 Marks)
Ans: Basalt is an igneous, volcanic rock that forms from the rapid cooling of basaltic magma. It is one of the most common volcanic rocks and plays a crucial role in understanding the Earth's mantle composition.
Mineralogy and Texture of Basalt
Mineralogy:
Primary Minerals:
Plagioclase Feldspar: The dominant mineral in basalt, typically rich in anorthite.
Pyroxene (Augite): Common in basalt, it is rich in iron and magnesium.
Olivine: Present in some basalt types, especially in more mafic basalts.
Magnetite: A common iron oxide mineral.
Texture:
Fine-grained Texture: Due to rapid cooling, basalt typically has a phaneritic texture where the minerals are too small to be easily seen with the naked eye.
Vesicular Texture: If gas bubbles are trapped during cooling, basalt may exhibit a vesicular texture (e.g., pumice).
Columnar Jointing: In some cases, basalt forms hexagonal columns as it cools and contracts.
Formation of Basaltic Magma in the Deep Earth
Partial Melting of the Mantle:
Basaltic magma forms through the partial melting of the Earth's upper mantle, which is composed of peridotite. The melting occurs due to decompression (reduction of pressure), increase in temperature, or the presence of volatiles (e.g., water) that lower the melting point.
Formation Process:
When mantle rocks experience decompression due to tectonic processes, like mid-ocean ridge spreading or hotspot activity, parts of the mantle melt to form basaltic magma.
As the magma rises towards the surface, it solidifies rapidly to form basalt when erupted at the surface, such as in shield volcanoes (e.g., Hawaii).
Example: Hawaiian Basalt: Erupted from shield volcanoes, rich in plagioclase feldspar and pyroxene.
Basalt is a fine-grained, mafic volcanic rock with a mineral composition dominated by plagioclase, pyroxene, and olivine. It forms from the partial melting of the Earth's mantle, contributing significantly to the oceanic crust.
(c) Discuss the process of magma generation in the Earth's interior and its causes. (20 Marks)
Ans: Magma generation in the Earth's interior is a critical process in the rock cycle, contributing to the formation of igneous rocks. Understanding how magma forms helps explain volcanic activity, plate tectonics, and the composition of the Earth’s crust.
Processes of Magma Generation
Decompression Melting:
Occurs at divergent boundaries or mantle plumes where tectonic plates are moving apart.
As the mantle material rises, the pressure decreases, allowing it to melt and form magma.
Example: Mid-ocean ridges and hotspots (e.g., Hawaii).
Flux Melting:
Occurs when water or other volatiles (e.g., CO2) are introduced into the mantle.
These volatiles lower the melting point of mantle rocks, facilitating magma formation.
Example: Subduction zones, where oceanic plates carrying water-rich sediments are forced downward, leading to magma formation.
Heat Transfer Melting:
Occurs when hot mantle material rises and comes into contact with cooler lithosphere, transferring heat and causing melting.
Example: Hotspots where mantle plumes rise beneath continental or oceanic crust.
Radiogenic Heating:
The decay of radioactive isotopes in the Earth’s mantle (e.g., Uranium, Thorium, and Potassium) generates heat, contributing to partial melting of the mantle.
Causes of Magma Generation
- Plate Tectonics: Subduction, divergent boundaries, and hotspot activity contribute significantly to magma formation.
- Mantle Convection: Convection currents in the mantle carry heat upwards, leading to partial melting of the mantle material.
- Temperature Increase: Mantle plumes bring hot mantle material to shallower depths, increasing the potential for melting.
Magma generation is a dynamic process influenced by tectonic movements, pressure changes, temperature variations, and the presence of volatiles. Understanding these processes is key to predicting volcanic activity and the formation of igneous rocks.
Q4:
(a) Discuss the various factors that control the composition of sandstone. (15 Marks)
Ans: Sandstone is a sedimentary rock primarily composed of mineral grains like quartz, feldspar, and other rock fragments, bound together by cementing material. The composition of sandstone varies based on several geological factors, including the source material, environment of deposition, and diagenetic processes.
Factors Controlling the Composition of Sandstone
Source Rock Composition:
The mineral composition of the source rocks directly influences the type of minerals found in sandstone. For example, if the source rock is granite, the resulting sandstone will be rich in quartz and feldspar.
Example: Sandstone derived from quartz-rich source rocks like granite or gneiss will primarily contain quartz grains.
Weathering:
The degree of weathering of source rocks affects the composition of the sandstone. Intense weathering tends to break down feldspar into clay minerals, making the sandstone more quartz-rich.
Example: Sandstones with high feldspar content typically come from areas with less intense weathering, like arid environments.
Transportation:
The degree of sorting during transportation (wind, water, or ice) influences the size and type of grains in the sandstone. Well-sorted sandstones typically contain uniform-sized grains of quartz, while poorly sorted sandstones have a more varied grain size.
Example: Wind-blown sandstones (like dune sands) tend to have fine grains of well-rounded quartz.
Depositional Environment:
The energy and mechanical processes in the depositional environment play a role in determining the composition. For instance, river and beach environments tend to produce well-rounded quartz sandstones, while deltaic or glacial environments may produce sandstones with more feldspar and lithic fragments.
Example: Sandstones formed in river beds are often rich in feldspar and lithic fragments, while beach sandstones are typically quartz-rich.
Cementation:
The type of cement (silica, calcium carbonate, or iron oxide) that binds the grains in the sandstone affects its final composition. Different cements may add or remove minerals, altering the overall composition.
Example: Sandstones cemented with iron oxide can have a reddish tint and may contain higher feldspar content.
Diagenesis:
After deposition, compaction and cementation during diagenesis can cause changes in mineral composition. For example, feldspar may undergo alteration into clay minerals due to chemical reactions during burial.
Example: Graywacke sandstones typically form under conditions of rapid burial and compaction.
The composition of sandstone is influenced by a combination of source material, weathering, transportation, depositional environment, and diagenetic processes. Each factor contributes to the mineralogical characteristics and texture of the resulting rock.
(b) What do you understand by facies model? Describe the facies and facies association produced in a fluvial environment. (15 Marks)
Ans: A facies model is a conceptual framework used by geologists to represent the lateral changes in rock types and their associated properties in a sedimentary basin. Facies models help in reconstructing past environments of deposition and understanding the evolution of sedimentary systems.
Facies Model
- A facies refers to a body of rock with specific characteristics (such as lithology, fossil content, and texture) that distinguish it from surrounding rocks.
- Facies models are used to predict the spatial distribution of sedimentary rocks, based on the geological processes that influenced their formation.
Facies in a Fluvial Environment
Floodplain Facies:
These are fine-grained, often siltstone or mudstone, deposited in low-energy conditions. They are usually rich in organic matter and exhibit bioturbation.
Example: Muddy deposits found in river floodplains.
Channel Facies:
Formed by the direct action of flowing water within a river channel, consisting of sandstones with cross-bedding and gravel.
Example: Braided river systems deposit coarse sandstones and conglomerates in the riverbed.
Point Bar Facies:
Accumulate on the inner bends of meandering rivers, consisting of fine to medium-grained sandstone with cross-bedding structures.
Example: Point bar deposits in meandering rivers, often containing fossilized plant remains or small-scale cross-stratification.
Levee Facies:
Occurs along the river banks, characterized by fine-grained sandstone and siltstone with occasional thin layers of clay. These deposits often show evidence of repeated flood events.
Example: River levee deposits with alternating sand and clay layers.
Facies Association in a Fluvial Environment
In a fluvial system, the typical facies association involves the transition from coarser grains (in channel facies) to finer grains (in floodplain and levee facies) as the water energy decreases.
Facies Relationships:
The point bar deposits (sandstone) are typically found adjacent to channel deposits (coarser sands or gravels).
The floodplain facies are typically overlying or adjacent to the point bar facies, with a gradation from sandstone to finer-grained siltstone or mudstone.
Facies models provide insights into ancient environments and their associated depositional features. In fluvial systems, the transition from coarse channel facies to finer floodplain facies helps reconstruct past river dynamics.
(c) What are heavy minerals? Describe methods of their separation and comment on the utility of heavy mineral suite in provenance interpretation. (20 Marks)
Ans: Heavy minerals are those minerals that have a high specific gravity, typically greater than 2.9. These minerals are often used in sedimentology and provenance studies to understand the origin and transport history of sedimentary rocks.
Heavy Minerals
- Examples of Heavy Minerals:-
- Zircon (ZrSiO4)
- Tourmaline (complex borosilicate)
- Rutile (TiO2)
- Ilmenite (FeTiO3)
- Hematite (Fe2O3)
- Spinel (MgAl2O4)
These minerals are chemically stable and are resistant to weathering, making them ideal indicators for provenance studies.
Methods of Separation of Heavy Minerals
- Density Separation: A common method where sediments are mixed with a dense liquid, such as bromoform or tetrabromoethane, to separate the heavy minerals from the light minerals.
- Magnetic Separation: Magnetic minerals (e.g., magnetite) can be separated from non-magnetic heavy minerals using a magnetic separator.
- Froth Flotation: This method involves using a flotation cell and adding chemical reagents to separate heavy minerals based on their surface properties.
- Gravity Separation: Heavy minerals are separated by their specific gravity using centrifugal force, often in a laboratory shaker.
Utility of Heavy Mineral Suite in Provenance Interpretation
Identification of Source Rock:
The suite of heavy minerals can indicate the source rock type, such as whether the sediments came from an igneous, metamorphic, or sedimentary source.
Example: The presence of zircon and tourmaline indicates a metamorphic source.
Tectonic Setting: The types and abundance of heavy minerals provide insights into the tectonic environment of the source area. For example, ultramafic minerals (e.g., chromite) indicate a cratonic or oceanic crust source.
Transport History: Heavy minerals are stable during transport and can help interpret the distance and duration of transport. High zircon content in a sediment may indicate long transport from the source.
Heavy minerals are crucial in sedimentary studies and provenance analysis, offering insights into source rocks, tectonic settings, and sediment transport. Their separation through methods like density separation and magnetic separation helps in understanding the geological history of sediments.
|
64 videos|135 docs
|
FAQs on UPSC Mains Answer PYQ 2024: Geology Paper 2 (Section- A) - Geology Optional for UPSC
| 1. What is the significance of Geology in the UPSC Mains exam? |  |
| 2. How can I effectively prepare for the Geology Paper in UPSC Mains? |  |
| 3. What are the main topics covered in the Geology syllabus for UPSC Mains? |  |
| 4. Are there any recommended books for Geology preparation for UPSC Mains? |  |
| 5. How is the Geology Paper evaluated in the UPSC Mains exam? |  |
















