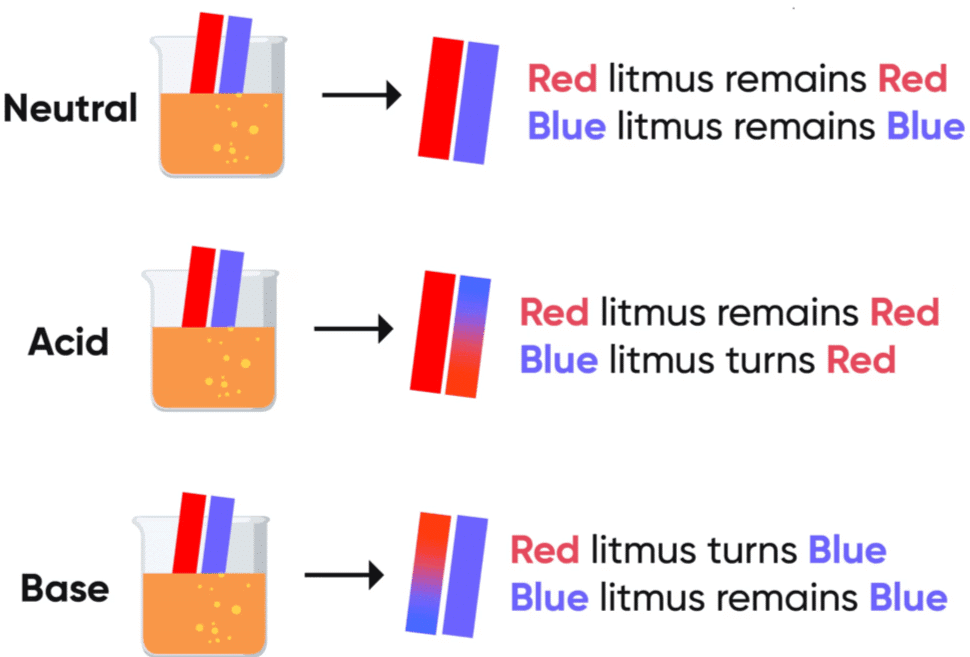
Activity 2.1: Testing with Olfactory and Chemical Indicators
- Collect the following solutions from the science laboratory– hydrochloric acid (HCl), sulphuric acid (H2SO4 ), nitric acid (HNO3 ), acetic acid (CH3COOH), sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2 ], potassium hydroxide (KOH), magnesium hydroxide [Mg(OH)2 ], and ammonium hydroxide (NH4OH).
- Put a drop of each of the above solutions on a watch-glass one by one and test with a drop of the indicators shown in Table.
- What change in colour did you observe with red litmus, blue litmus, phenolphthalein and methyl orange solutions for each of the solutions taken?
- Tabulate your observations in Table.
Observations:
| Solution | Red Litmus Solution | Blue Litmus Solution | Phenolphthalein Solution | Methyl Orange Solution |
|---|---|---|---|---|
| Hydrochloric Acid (HCl) | Remains Red | Turns Red | Colourless | Red |
| Sulfuric Acid (H₂SO₄) | Remains Red | Turns Red | Colourless | Red |
| Nitric Acid (HNO₃) | Remains Red | Turns Red | Colourless | Red |
| Acetic Acid (CH₃COOH) | Remains Red | Turns Red | Colourless | Red |
| Sodium Hydroxide (NaOH) | Turns Blue | Remains Blue | Pink | Yellow |
| Calcium Hydroxide (Ca(OH)₂) | Turns Blue | Remains Blue | Pink | Yellow |
| Potassium Hydroxide (KOH) | Turns Blue | Remains Blue | Pink | Yellow |
| Magnesium Hydroxide (Mg(OH)₂) | Turns Blue | Remains Blue | Pink | Yellow |
| Ammonium Hydroxide (NH₄OH) | Turns Blue | Remains Blue | Pink | Yellow |
Activity 2.2: Testing for Olfactory Indicators Using Onion, Vanilla, and Clove
- Take some finely chopped onions in a plastic bag along with some strips of clean cloth. Tie up the bag tightly and leave overnight in the fridge. The cloth strips can now be used to test for acids and bases.
- Take two of these cloth strips and check their odour.
- Keep them on a clean surface and put a few drops of dilute HCl solution on one strip and a few drops of dilute NaOH solution on the other
- Rinse both cloth strips with water and again check their odour.
- Note your observations.
- Now take some dilute vanilla essence and clove oil and check their odour.
- Take some dilute HCl solution in one test tube and dilute NaOH solution in another. Add a few drops of dilute vanilla essence to both test tubes and shake well. Check the odour once again and record changes in odour, if any.
- Similarly, test the change in the odour of clove oil with dilute HCl and dilute NaOH solutions and record your observations.
Observations:
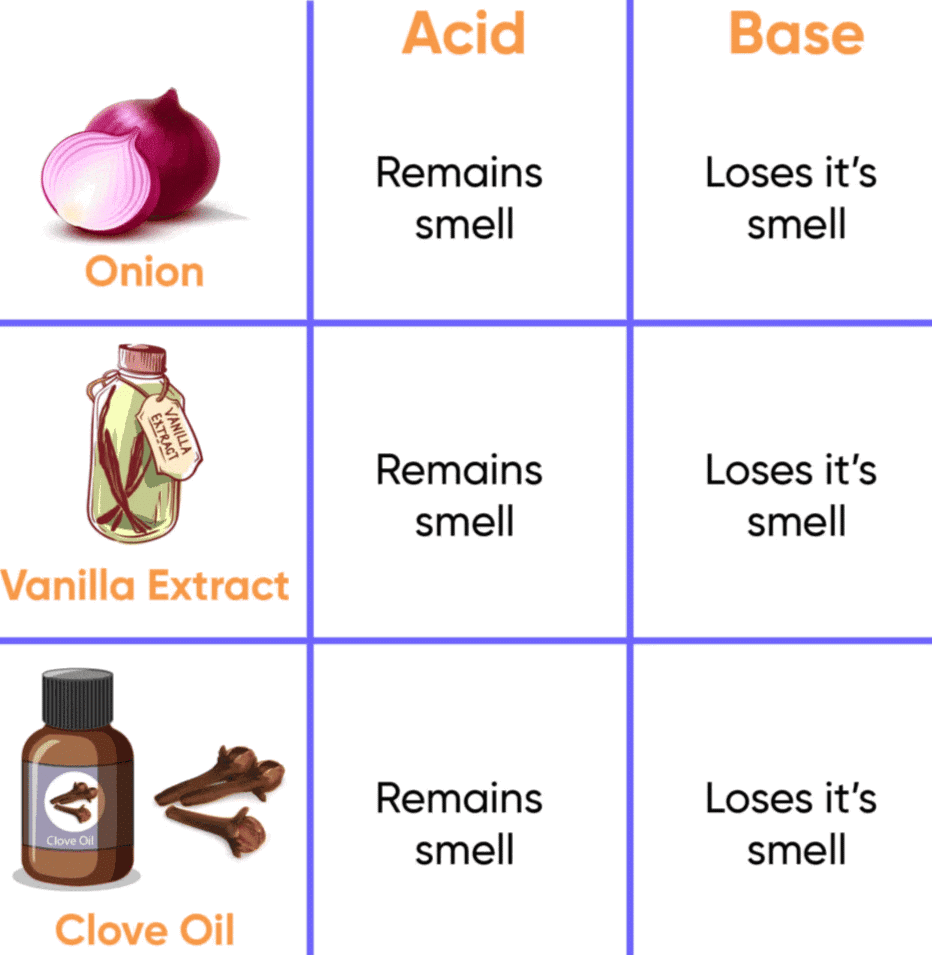
Ques : Which of these – vanilla, onion and clove, can be used as olfactory indicators on the basis of your observations?
Ans:
Onion can be used as an olfactory indicator because its odour changes in the presence of an acid (HCl) or base (NaOH), with the odour intensity being affected differently by the two solutions.
Vanilla essence and clove oil can also be used as olfactory indicators, though their changes in odour are not as significant or distinct as those of onion. Both show noticeable changes in odour upon reaction with acids and bases, which makes them potential olfactory indicators as well.
Thus, onion is the most suitable olfactory indicator based on the observations.
Activity 2.3: Reaction of Zinc with Dilute Acids
CAUTION: This activity needs the teacher’s assistance.
- Set the apparatus as shown in Fig.
- Take about 5 mL of dilute sulphuric acid in a test tube and add a few pieces of zinc granules to it.
- What do you observe on the surface of zinc granules?
- Pass the gas being evolved through the soap solution.
- Why are bubbles formed in the soap solution?
- Take a burning candle near a gas filled bubble.
- What do you observe?
- Repeat this Activity with some more acids like HCl, HNO3 and CH3COOH.
- Are the observations in all the cases the same or different?
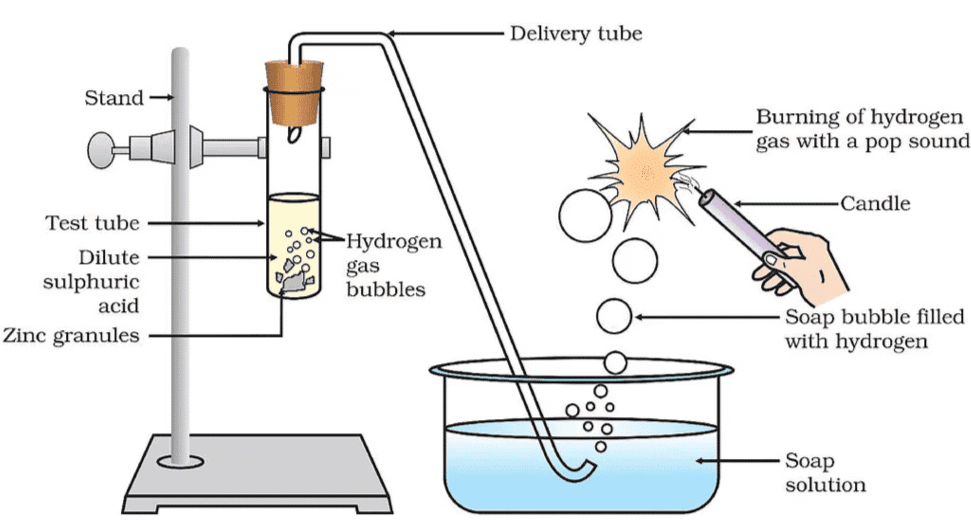 Reaction of zinc granules with dilute sulphuric acid and testing hydrogen gas by burning
Reaction of zinc granules with dilute sulphuric acid and testing hydrogen gas by burning
Observations:
On the surface of zinc granules: You will observe bubbles forming, which is hydrogen gas being evolved.
Bubbles in soap solution: The bubbles form because hydrogen gas is passing through the soap solution, creating a foam of hydrogen bubbles.
When a burning candle is brought near a bubble: The hydrogen gas inside the bubble burns with a pop sound, confirming the presence of hydrogen.
- With other acids (HCl, HNO₃, CH₃COOH): You will still observe the evolution of hydrogen gas in all cases. The intensity of the reaction might be stronger with HCl due to its stronger acidic nature compared to acetic acid (CH₃COOH), which is weaker.
Reaction of Zinc with Hydrochloric Acid (HCl): Zn (s)+2HCl (aq)→ZnCl₂ (aq)+H₂ (g)
Reaction of Zinc with Sulfuric Acid (H₂SO₄): Zn (s)+H₂SO₄ (aq)→ZnSO₄ (aq)+H₂ (g)
Reaction of Zinc with Acetic Acid (CH₃COOH): Zn (s)+2CH₃COOH (aq)→Zn(CH₃COO)₂ (aq)+H₂ (g)
Conclusion:
In this activity, zinc reacts with various dilute acids to release hydrogen gas (H₂). The reaction can be confirmed by passing the gas through a soap solution to form bubbles and bringing a burning candle near the bubbles to observe the pop sound.
The observations in all cases are similar, as zinc reacts with acids to produce hydrogen gas, but the rate of reaction can vary with the strength of the acid.
Activity 2.4: Reaction of Zinc with NaOH Solution
- Place a few pieces of granulated zinc metal in a test tube.
- Add 2 mL of sodium hydroxide solution and warm the contents of the test tube.
- Repeat the rest of the steps as in Activity and record your observations.
Observations:
When zinc reacts with sodium hydroxide solution, bubbles of gas will form.
The gas produced is hydrogen gas (H₂).
When the gas passes through the soap solution, bubbles will form because the hydrogen gas is trapped in the soap film.
When you bring a burning candle near the bubbles, the hydrogen gas burns with a pop sound, confirming the presence of hydrogen gas.
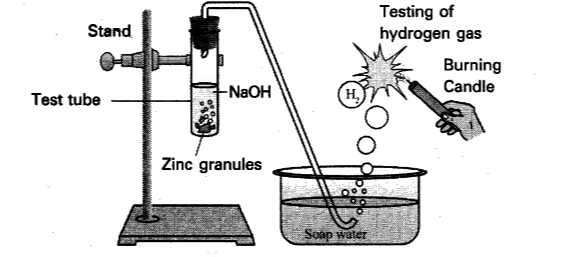 Reaction of Zinc with Sodium Hydroxide SolutionChemical Reaction: Zn (s)+2NaOH (aq)→Na₂ZnO₂ (aq)+H₂ (g)
Reaction of Zinc with Sodium Hydroxide SolutionChemical Reaction: Zn (s)+2NaOH (aq)→Na₂ZnO₂ (aq)+H₂ (g)
Explanation:
- Zinc reacts with sodium hydroxide (NaOH) to form sodium zincate (Na₂ZnO₂) and hydrogen gas (H₂).
- The production of hydrogen gas is similar to the reaction with acids, and it can be tested by observing the bubbles and the pop sound when exposed to a burning candle.
Conclusion:
- The reaction between zinc and sodium hydroxide solution produces hydrogen gas (H₂), which can be confirmed by the formation of bubbles in the soap solution and the pop sound when brought near a burning candle.
- This is a redox reaction where zinc is oxidized and hydrogen ions from sodium hydroxide are reduced to hydrogen gas.
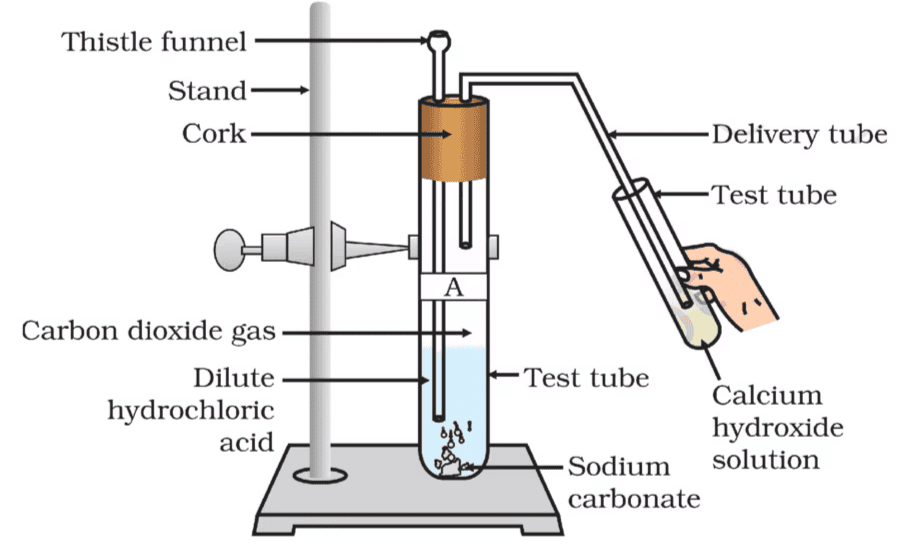
Activity 2.5: Reaction of Sodium Carbonate and Sodium Bicarbonate with HCl
- Take two test tubes, label them as A and B.
- Take about 0.5 g of sodium carbonate (Na2CO3 ) in test tube A and about 0.5 g of sodium hydrogencarbonate (NaHCO3 ) in test tube B.
- Add about 2 mL of dilute HCl to both the test tubes.
- What do you observe?
- Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in Fig. and record your observations.
Observations:
1. Test Tube A (Sodium Carbonate + HCl):
- When sodium carbonate (Na₂CO₃) reacts with hydrochloric acid (HCl), it produces carbon dioxide (CO₂) gas.
- You will observe bubbling in both test tubes due to the evolution of gas.
- Passing the gas through lime water: The gas reacts with lime water and forms a milky white precipitate of calcium carbonate (CaCO₃), indicating the presence of carbon dioxide (CO₂) gas.
Reaction:
Na₂CO₃ (aq)+2HCl (aq)→2NaCl (aq)+H₂O (l)+CO₂ (g)
CO₂ (g)+Ca(OH)₂ (aq)→CaCO₃ (s)+H₂O (l)
2. Test Tube B (Sodium Bicarbonate + HCl):
- When sodium bicarbonate (NaHCO₃) reacts with hydrochloric acid (HCl), it also produces carbon dioxide (CO₂) gas.
- You will observe bubbling in this test tube as well due to gas production.
- Passing the gas through lime water: The gas reacts with lime water and forms a milky white precipitate of calcium carbonate (CaCO₃), again confirming the presence of carbon dioxide (CO₂) gas.
Reaction:
NaHCO₃ (aq)+HCl (aq)→NaCl (aq)+H₂O (l)+CO₂ (g)CO₂ (g)+Ca(OH)₂ (aq)→CaCO₃ (s)+H₂O (l)
Conclusion:
- Both sodium carbonate (Na₂CO₃) and sodium bicarbonate (NaHCO₃) react with dilute hydrochloric acid (HCl) to produce carbon dioxide (CO₂) gas.
- The formation of a milky precipitate in lime water confirms that carbon dioxide (CO₂) gas is produced in both reactions.
- The key difference between the reactions of sodium carbonate and sodium bicarbonate is that sodium bicarbonate reacts more readily with HCl and produces CO₂ at a lower temperature.
Activity 2.6: Observing the Effect of Acid and Base on Phenolphthalein Solution
- Take about 2 mL of dilute NaOH solution in a test tube and add two drops of phenolphthalein solution.
- What is the colour of the solution?
- Add dilute HCl solution to the above solution drop by drop.
- Is there any colour change for the reaction mixture?
- Why did the colour of phenolphthalein change after the addition of an acid?
- Now add a few drops of NaOH to the above mixture.
- Does the pink colour of phenolphthalein reappear?
- Why do you think this has happened?
Observations:
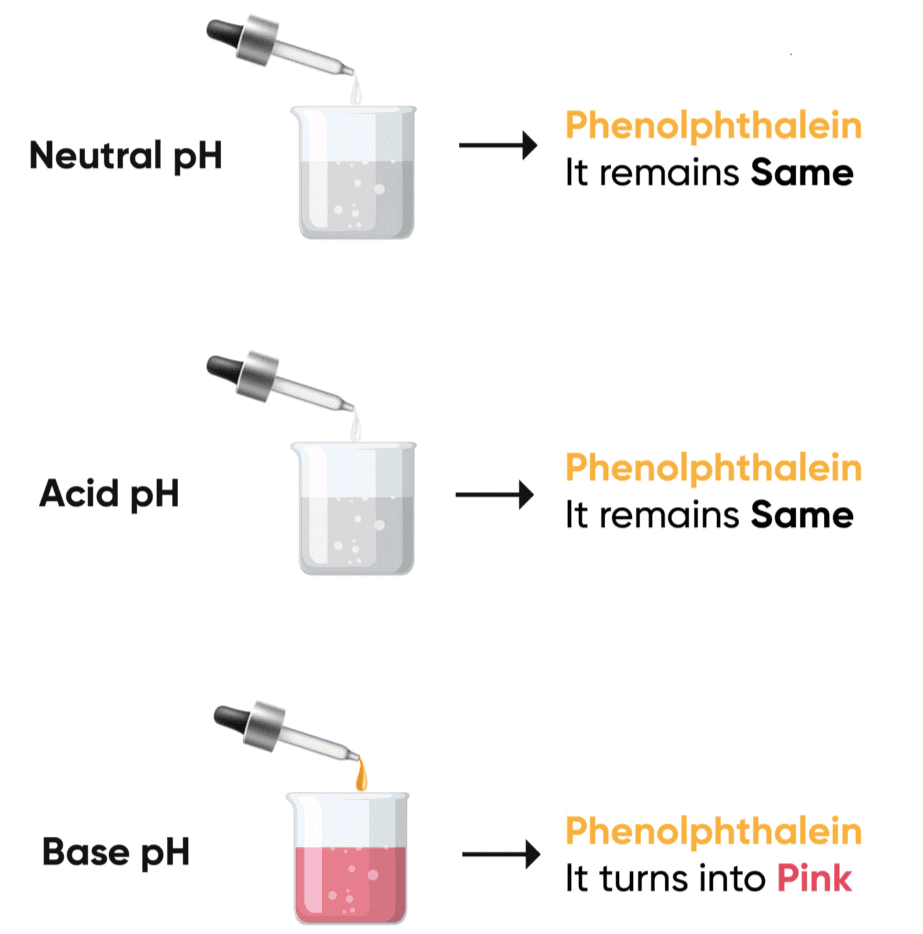
- The solution is pink at the start when NaOH is present because phenolphthalein is pink in basic solutions.
- As dilute HCl is added, the solution turns colorless because the pH decreases, making the solution acidic.
- When NaOH is added again, the solution turns pink as the pH increases and becomes basic once more.
Conclusion:
Phenolphthalein is an indicator that changes color depending on the pH of the solution: Pink in basic solutions (pH > 7) and Colourless in acidic solutions (pH < 7). The change in colour occurs due to the change in the pH level as acid or base is added. This activity demonstrates how the pH of a solution can be monitored using phenolphthalein as an indicator.
Activity 2.7: Reaction of Copper Oxide with HCl
- Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly while stirring.
- Note the colour of the solution. What has happened to the copper oxide?
Observations:
Before the reaction, copper oxide is a black solid.
As dilute hydrochloric acid (HCl) is added and stirred, the black color of copper oxide disappears, and a blue or green solution forms.
Copper oxide reacts with hydrochloric acid to form copper chloride (CuCl₂) and water (H₂O), which dissolves in the acid to form a blue/green solution.
Chemical Reaction: CuO (s)+2HCl (aq)→CuCl₂ (aq)+H₂O (l)
Conclusion:
Copper oxide is a basic oxide. When it reacts with dilute hydrochloric acid (HCl), it forms copper chloride (CuCl₂), a soluble salt, and water.
The color change from black (copper oxide) to blue/green (copper chloride solution) indicates that the copper oxide has dissolved in the acid to form a soluble copper salt.
Activity 2.8: Testing the Electrical Conductivity of Different Solutions
- Take solutions of glucose, alcohol, hydrochloric acid, sulphuric acid, etc.
- Fix two nails on a cork, and place the cork in a 100 mL beaker.
- Connect the nails to the two terminals of a 6 volt battery through a bulb and a switch, as shown in Fig.
- Now pour some dilute HCl in the beaker and switch on the current.
- Repeat with dilute sulphuric acid.
- What do you observe?
- Repeat the experiment separately with glucose and alcohol solutions. What do you observe now?
- Does the bulb glow in all cases?
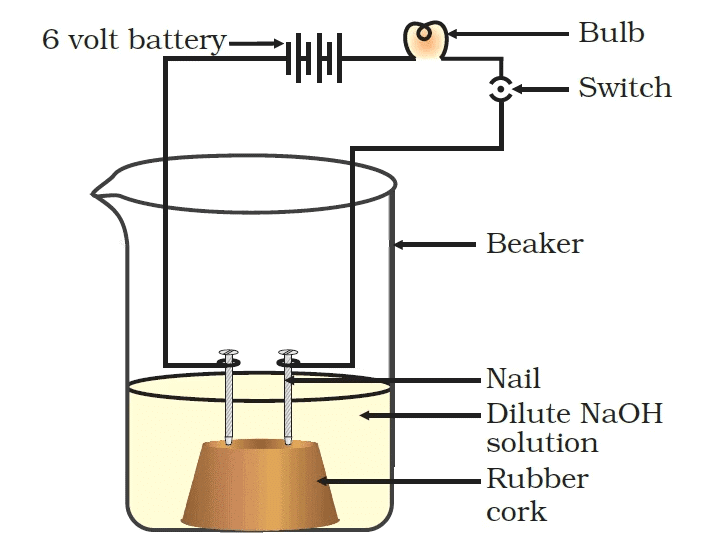
Observations:
With dilute HCl: When dilute HCl is added, the bulb glows, indicating that the solution conducts electricity. HCl dissociates into H⁺ ions and Cl⁻ ions, which are charged particles that can carry current, making it a good conductor of electricity.
With dilute sulfuric acid: When dilute sulfuric acid is added, the bulb glows brightly, showing that sulfuric acid also conducts electricity. Sulfuric acid (H₂SO₄) dissociates into H⁺ ions and SO₄²⁻ ions, which also allow the flow of current.
With glucose solution: When glucose solution is added, the bulb does not glow. This is because glucose does not dissociate into ions in solution, and therefore does not conduct electricity. It is a non-electrolyte.
With alcohol solution: When alcohol solution is added, the bulb does not glow. Like glucose, alcohol is a non-electrolyte, meaning it does not dissociate into ions and therefore does not conduct electricity.
Conclusion:
The bulb glows when the solution is a good conductor of electricity, which happens in acidic solutions like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄). These are electrolytes that dissociate into ions.
The bulb does not glow with glucose or alcohol solutions, indicating that these are non-electrolytes that do not dissociate into ions and thus do not conduct electricity.
Activity 2.9: Reaction of Sodium Chloride with Concentrated Sulphuric Acid
- Take about 1g solid NaCl in a clean and dry test tube and set up the apparatus as shown in Fig.
- Add some concentrated sulphuric acid to the test tube.
- What do you observe? Is there a gas coming out of the delivery tube?
- Test the gas evolved successively with dry and wet blue litmus paper.
- In which case does the litmus paper change colour?
- On the basis of the above Activity, what do you infer about the acidic character of: (i) dry HCl gas (ii) HCl solution?
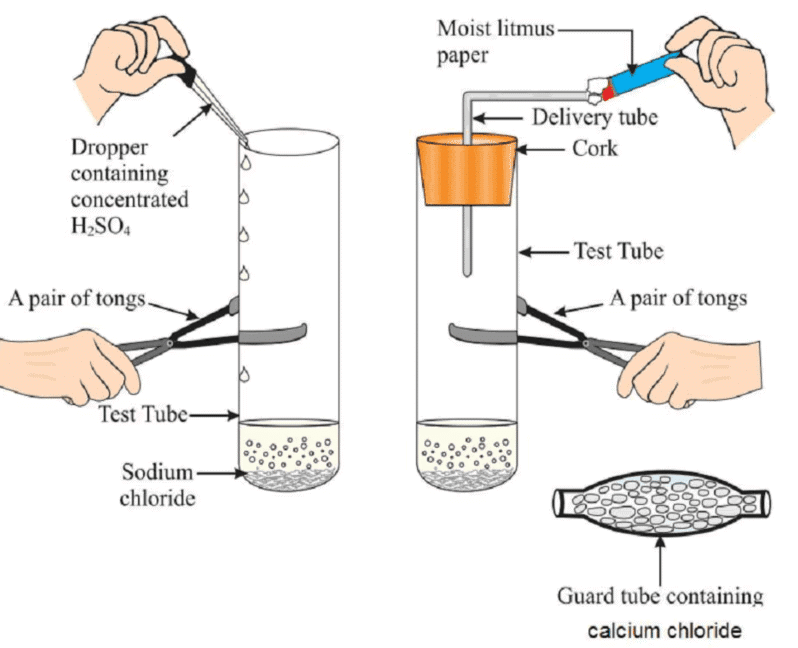 Preparation of HCl gas
Preparation of HCl gas
Observations:
Gas evolved: The gas evolved is hydrogen chloride (HCl) gas, which is acidic in nature.
Litmus test:
(i) Dry blue litmus paper turns red, indicating that HCl gas is acidic.
(ii) Wet blue litmus paper also turns red, further confirming the acidic nature of the evolved gas
Inferences:
Acidic character of dry HCl gas: The dry HCl gas is acidic as it turns blue litmus paper red, indicating that it has acidic properties even in the gaseous state.
Acidic character of HCl solution: HCl solution (formed when HCl gas dissolves in water) is also acidic, as it turns both dry and wet blue litmus paper red. This confirms that the solution contains hydrogen ions (H⁺), which are responsible for its acidic nature.
Conclusion:
- The reaction of sodium chloride (NaCl) with concentrated sulphuric acid (H₂SO₄) produces hydrogen chloride (HCl) gas.
- HCl gas is acidic, as demonstrated by its ability to turn blue litmus paper red, both in dry and wet conditions.
- The acidic properties of dry HCl gas and HCl solution are confirmed through the litmus test, showing that both exhibit acidic behaviour.
Activity 2.10: Temperature Changes during the Addition of Acid and Base to Water
- Take 10 mL water in a beaker.
- Add a few drops of concentrated H2SO4 to it and swirl the beaker slowly.
- Touch the base of the beaker. n Is there a change in temperature?
- Is this an exothermic or endothermic process?
- Repeat the above Activity with sodium hydroxide pellets and record your observations
Observations:
With Concentrated H₂SO₄ (Sulphuric Acid):
After adding concentrated sulfuric acid to water, you will feel an increase in temperature. This is because the reaction is exothermic, meaning heat is released during the process.
Conclusion: The process is exothermic, as there is a rise in temperature when sulfuric acid is added to water.
With Sodium Hydroxide (NaOH) Pellets:
When NaOH pellets are added to water, you will again feel an increase in temperature. The dissolution of sodium hydroxide in water is an exothermic reaction, where heat is released.
Conclusion: The process is exothermic, as there is a rise in temperature when sodium hydroxide dissolves in water.
Conclusion:
Both the reactions, when concentrated sulphuric acid and sodium hydroxide are added to water, are exothermic reactions, as they both release heat and cause an increase in temperature.
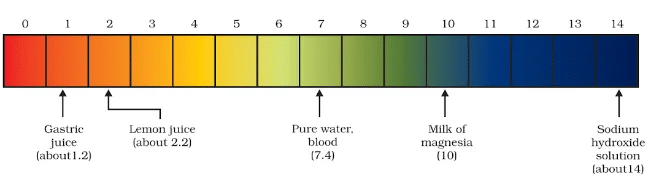
Activity 2.11: Testing the pH of Different Solutions
- Test the pH values of solutions given in Table.
- Record your observations.
- What is the nature of each substance on the basis of your observations?
Observations:
| Solution | pH Value | Nature of Solution |
|---|---|---|
| Hydrochloric Acid (HCl) | ~1-2 | Acidic |
| Sodium Hydroxide (NaOH) | ~13-14 | Basic |
| Ammonium Hydroxide (NH₄OH) | ~11 | Basic |
| Vinegar (Acetic Acid) | ~4-5 | Acidic |
| Water | 7 | Neutral |
| Lemon Juice | ~2-3 | Acidic |
| Sodium Carbonate (Na₂CO₃) | ~11 | Basic |
Conclusion:
Acidic Solutions: These have pH values less than 7. Examples from the above list include Hydrochloric acid (HCl), Vinegar, and Lemon juice.
Basic Solutions: These have pH values greater than 7. Examples include Sodium hydroxide (NaOH) and Ammonium hydroxide (NH₄OH).
Neutral Solution: The solution with a pH value of 7 is Water, which is neutral.
Activity 2.12: Determining the pH of Soil Filtrate
- Put about 2 g soil in a test tube and add 5 mL water to it.
- Shake the contents of the test tube.
- Filter the contents and collect the filtrate in a test tube.
- Check the pH of this filtrate with the help of universal indicator paper.
- What can you conclude about the ideal soil pH for the growth of plants in your region?
Observations:
The color of the universal indicator paper will indicate the pH of the soil filtrate.
If the pH is between 6 and 7, the soil is neutral.
If the pH is below 6, the soil is acidic.
If the pH is above 7, the soil is basic (alkaline).
Conclusion:
Based on the pH of the filtrate, you can conclude the ideal pH range for soil in your region.
Neutral soils (pH 6-7) are generally ideal for most plants, as they allow for optimal nutrient availability.
Slightly acidic or basic soils (pH around 6-6.5 or 7.5-8) may be suitable for certain plants, but extreme pH values (very acidic or very alkaline soils) may hinder plant growth.
In your region, if the soil is found to be too acidic or too basic, you may need to adjust the soil pH using appropriate soil amendments (e.g., adding lime to raise pH in acidic soil, or adding sulfur to lower pH in alkaline soil).
Activity 2.13: Writing Chemical Formulae of Salts and Identifying Their Families
- Write the chemical formulae of the salts given below. Potassium sulphate, sodium sulphate, calcium sulphate, magnesium sulphate, copper sulphate, sodium chloride, sodium nitrate, sodium carbonate and ammonium chloride.
- Identify the acids and bases from which the above salts may be obtained.
- Salts having the same positive or negative radicals are said to belong to a family. For example, NaCl and Na2SO4 belong to the family of sodium salts. Similarly, NaCl and KCl belong to the family of chloride salts. How many families can you identify among the salts given in this Activity?
Chemical Formulae of the Given Salts:
- Potassium sulfate: K₂SO₄
- Sodium sulfate: Na₂SO₄
- Calcium sulfate: CaSO₄
- Magnesium sulfate: MgSO₄
- Copper sulfate: CuSO₄
- Sodium chloride: NaCl
- Sodium nitrate: NaNO₃
- Sodium carbonate: Na₂CO₃
- Ammonium chloride: NH₄Cl
Acids and Bases from Which These Salts Are Obtained:
1. Potassium sulfate (K₂SO₄): Obtained from potassium hydroxide (KOH) and sulfuric acid (H₂SO₄).2. Sodium sulfate (Na₂SO₄): Obtained from sodium hydroxide (NaOH) and sulfuric acid (H₂SO₄).
3. Calcium sulfate (CaSO₄): Obtained from calcium hydroxide (Ca(OH)₂) and sulfuric acid (H₂SO₄).
4. Magnesium sulfate (MgSO₄): Obtained from magnesium hydroxide (Mg(OH)₂) and sulfuric acid (H₂SO₄).
5. Copper sulfate (CuSO₄): Obtained from copper (Cu) and sulfuric acid (H₂SO₄).
6. Sodium chloride (NaCl): Obtained from sodium hydroxide (NaOH) and hydrochloric acid (HCl).
7. Sodium nitrate (NaNO₃): Obtained from sodium hydroxide (NaOH) and nitric acid (HNO₃).
8. Sodium carbonate (Na₂CO₃): Obtained from sodium hydroxide (NaOH) and carbonic acid (H₂CO₃).
9. Ammonium chloride (NH₄Cl): Obtained from ammonium hydroxide (NH₄OH) and hydrochloric acid (HCl).
Identifying Families of Salts:
Salts can be grouped into families based on common positive or negative radicals (ions). Here are the families based on the salts provided:
Sodium Salts Family:
NaCl (Sodium chloride)
Na₂SO₄ (Sodium sulfate)
NaNO₃ (Sodium nitrate)
Na₂CO₃ (Sodium carbonate)
- These salts all contain the common sodium (Na⁺) ion.
Sulfate Salts Family:
K₂SO₄ (Potassium sulfate)
Na₂SO₄ (Sodium sulfate)
CaSO₄ (Calcium sulfate)
MgSO₄ (Magnesium sulfate)
CuSO₄ (Copper sulfate)
- These salts all contain the common sulfate (SO₄²⁻) radical.
Chloride Salts Family:
NaCl (Sodium chloride)
KCl (Potassium chloride, though not part of the list here, it would belong to this family as well)
- These salts all contain the common chloride (Cl⁻) radical.
Conclusion:
Families Identified:
Sodium salts family (Na⁺)
Sulfate salts family (SO₄²⁻)
Chloride salts family (Cl⁻)
By identifying the common radicals or ions in the salts, we have classified the salts into 3 families: sodium salts, sulphate salts, and chloride salts.
Activity 2.14: Testing the Solubility, pH, and Nature of Various Salts
- Collect the following salt samples – sodium chloride, potassium nitrate, aluminium chloride, zinc sulphate, copper sulphate, sodium acetate, sodium carbonate and sodium hydrogencarbonate (some other salts available can also be taken).
- Check their solubility in water (use distilled water only).
- Check the action of these solutions on litmus and find the pH using a pH paper.
- Which of the salts are acidic, basic or neutral?
- Identify the acid or base used to form the salt.
- Report your observations in Table.
Observations:
| Salt Sample | Solubility in Water | Action on Litmus Paper | pH | Nature of Solution | Acid/Base Used to Form the Salt |
|---|---|---|---|---|---|
| Sodium chloride (NaCl) | Soluble | No change (Neutral) | 7 | Neutral | Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) |
| Potassium nitrate (KNO₃) | Soluble | No change (Neutral) | 7 | Neutral | Nitric acid (HNO₃) + Potassium hydroxide (KOH) |
| Aluminium chloride (AlCl₃) | Soluble | Turns blue (Basic) | >7 | Basic | Hydrochloric acid (HCl) + Aluminium hydroxide (Al(OH)₃) |
| Zinc sulfate (ZnSO₄) | Soluble | No change (Neutral) | 7 | Neutral | Sulfuric acid (H₂SO₄) + Zinc hydroxide (Zn(OH)₂) |
| Copper sulfate (CuSO₄) | Soluble | No change (Neutral) | 7 | Neutral | Sulfuric acid (H₂SO₄) + Copper hydroxide (Cu(OH)₂) |
| Sodium acetate (CH₃COONa) | Soluble | Turns red (Acidic) | <7 | Acidic | Acetic acid (CH₃COOH) + Sodium hydroxide (NaOH) |
| Sodium carbonate (Na₂CO₃) | Soluble | Turns red (Basic) | >7 | Basic | Carbonic acid (H₂CO₃) + Sodium hydroxide (NaOH) |
| Sodium bicarbonate (NaHCO₃) | Soluble | Turns red (Basic) | >7 | Basic | Carbonic acid (H₂CO₃) + Sodium hydroxide (NaOH) |
Conclusion:
Neutral Solutions: Sodium chloride (NaCl), Potassium nitrate (KNO₃), Zinc sulfate (ZnSO₄), and Copper sulfate (CuSO₄) are neutral as they neither change the litmus paper color nor have a pH significantly above or below 7.
Acidic Solutions: Sodium acetate (CH₃COONa) shows acidic behavior with a pH less than 7 and turns blue litmus paper red.
Basic Solutions: Sodium carbonate (Na₂CO₃), Sodium bicarbonate (NaHCO₃), and Aluminium chloride (AlCl₃) behave as basic solutions, with a pH greater than 7 and turning red litmus paper blue.
Families Identified:
Sodium salts family: NaCl, Na₂CO₃, NaHCO₃, CH₃COONa
Chloride salts family: NaCl, AlCl₃
Sulfate salts family: ZnSO₄, CuSO₄
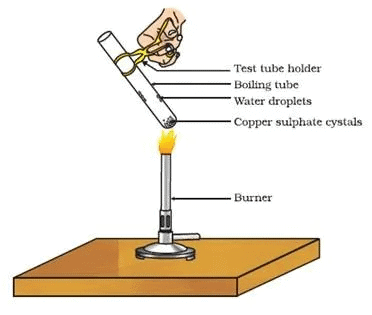
Activity 2.15: Heating Copper Sulphate and Observing Colour Change
- Heat a few crystals of copper sulphate in a dry boiling tube.
- What is the colour of the copper sulphate after heating?
- Do you notice water droplets in the boiling tube? Where have these come from?
- Add 2-3 drops of water on the sample of copper sulphate obtained after heating.
- What do you observe? Is the blue colour of copper sulphate restored?
Observations:
Before heating: Copper sulfate crystals are blue in color.
After heating: Upon heating, the copper sulfate crystals lose their water of crystallization and turn into a white powder, known as anhydrous copper sulfate.
Water droplets: After heating, you will observe water droplets inside the boiling tube, which is the water of crystallization evaporating from the copper sulfate.
Restoration of blue color: When you add a few drops of water to the anhydrous copper sulfate, the blue color is restored, indicating that the copper sulfate has absorbed the water and returned to its hydrated form, copper sulfate pentahydrate (CuSO₄·5H₂O).
Conclusion:
Heating copper sulfate removes its water of crystallization, causing it to change from blue to white (anhydrous form).
Upon adding water, the blue color is restored, demonstrating the reversible nature of the hydration process of copper sulfate.