Cheat Sheet: Acids, Bases and Salts | Science Class 10 PDF Download
Understanding Acids and Bases
1. Acids: Sour in taste, turn blue litmus red. Examples: HCl, H₂SO₄, HNO₃, CH₃COOH.
2. Bases: Bitter in taste, turn red litmus blue. Examples: NaOH, Ca(OH)₂, KOH, Mg(OH)₂, NH₄OH.
3. Indicators: Substances that show different colors in acidic and basic solutions.
- Natural Indicators: Litmus (purple in neutral, red in acid, blue in base), turmeric, red cabbage, flower petals (e.g., Hydrangea, Petunia, Geranium).
- Synthetic Indicators: Phenolphthalein (colorless in acid, pink in base), methyl orange (red in acid, yellow in base).
- Olfactory Indicators: Change odor in acidic or basic media (e.g., onion, vanilla, clove).
Chemical Properties of Acids and Bases
1. Reaction with Metals
(a) Acids: React with metals to produce salt and hydrogen gas.
- General Reaction: Acid + Metal → Salt + H₂(g)
- Example: H₂SO₄(aq) + Zn(s) → ZnSO₄(aq) + H₂(g)
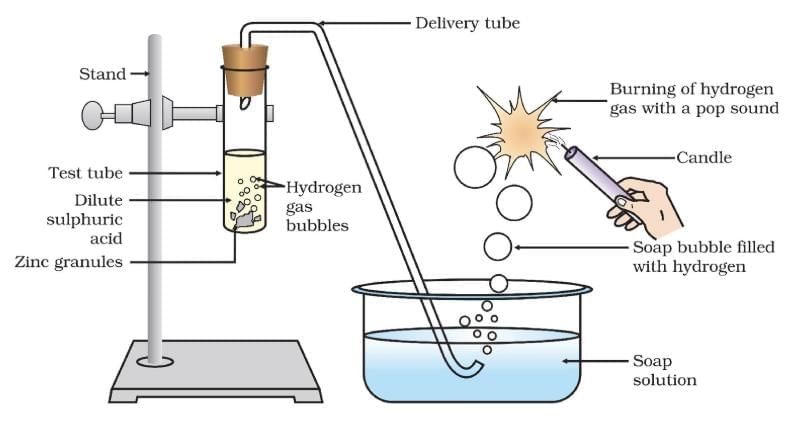
Note: Test for H₂: Burns with a pop sound near a burning candle.
(b) Bases: Some bases (e.g., NaOH) react with certain metals (e.g., Zn) to form salt and hydrogen gas.
- Example: 2NaOH(aq) + Zn(s) → Na₂ZnO₂(s) + H₂(g) (Sodium zincate).
2. Reaction with Metal Carbonates/Hydrogencarbonates
Acids: Produce salt, carbon dioxide, and water.
Metal carbonate/hydrogencarbonate + Acid → Salt + CO₂(g) + H₂O(l)
- Na₂CO₃(s) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
- NaHCO₃(s) + HCl(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
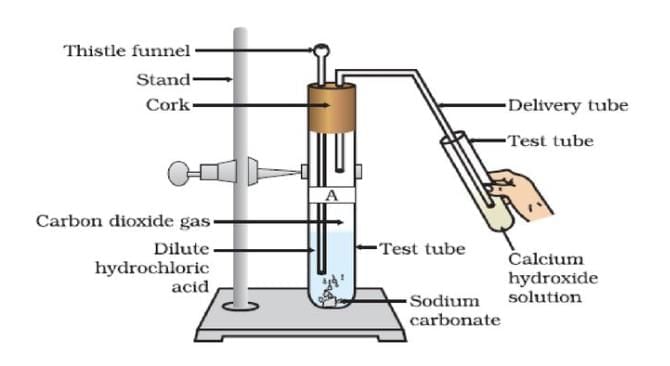
Note: Test for CO₂: Turns lime water [Ca(OH)₂] milky due to CaCO₃ formation; excess CO₂ forms soluble Ca(HCO₃)₂.
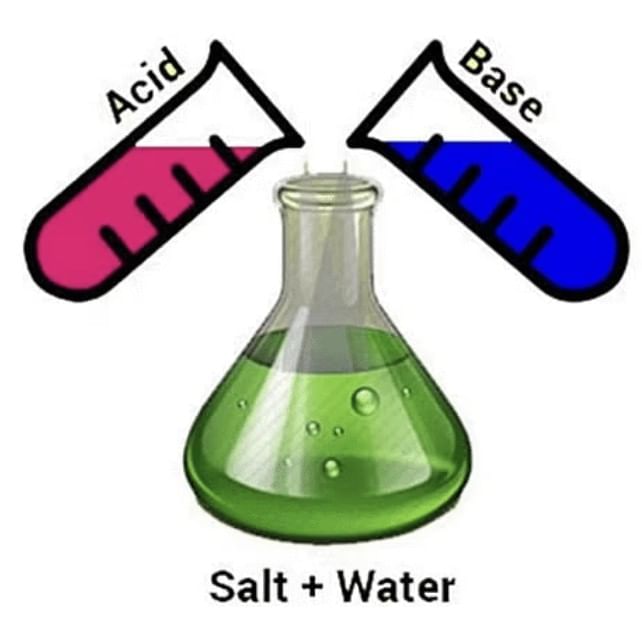
3. Reaction of Acids and Bases (Neutralization)
Acid reacts with base to form salt and water.
- General Reaction: Acid + Base → Salt + H₂O
- Example: NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
- Ionic Reaction: H⁺(aq) + OH⁻(aq) → H₂O(l)
- Observation: Phenolphthalein turns pink in base, colorless in acid.
4. Reaction of Metallic Oxides with Acids
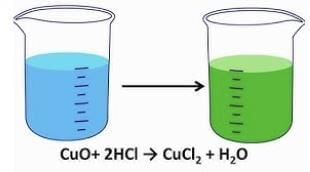
Metallic Oxides: Act as bases, react with acids to form salt and water.
- Example: CuO(s) + 2HCl(aq) → CuCl₂(aq) + H₂O(l) (Blue-green solution).
- Conclusion: Metallic oxides are basic oxides.
5. Reaction of Non-Metallic Oxides with Bases
Non-Metallic Oxides: Act as acids, react with bases to form salt and water.
- Example: Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
- Conclusion: Non-metallic oxides are acidic.
Commonality in Acids and Bases
(a) Acids: Produce H⁺(aq) or H₃O⁺ (hydronium ions) in water, responsible for acidic properties.
- Example: HCl + H₂O → H₃O⁺ + Cl⁻
- Dry HCl gas does not show acidic behavior (no H⁺ ions without water).
(b) Bases: Produce OH⁻(aq) ions in water; soluble bases are called alkalis.
- Examples: NaOH(s) → Na⁺(aq) + OH⁻(aq), KOH(s) → K⁺(aq) + OH⁻(aq)
- Conductivity: Acidic and basic solutions conduct electricity due to H⁺/H₃O⁺ or OH⁻ ions.
Non-acidic hydrogen-containing compounds (e.g., glucose, alcohol) do not produce H⁺ ions, so they don’t conduct electricity.
Strength of Acids and Bases
(a) pH Scale: Measures H⁺ ion concentration (0–14).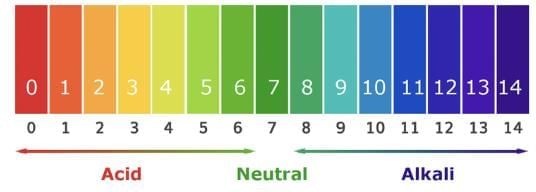
- pH < 7: Acidic (higher H⁺ concentration, lower pH).
- pH = 7: Neutral (e.g., distilled water).
- pH > 7: Basic (higher OH⁻ concentration).
(b) Universal Indicator: Shows different colors for different pH values.
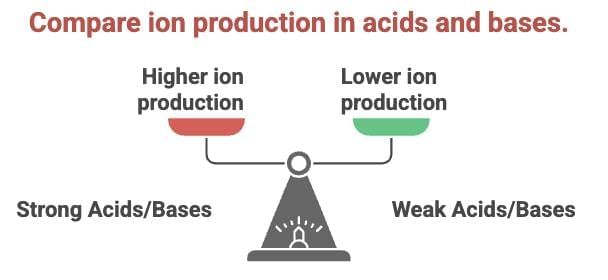
(c) Strong vs. Weak:
- Strong acids (e.g., HCl) produce more H⁺ ions; weak acids (e.g., CH₃COOH) produce fewer.
- Strong bases (e.g., NaOH) produce more OH⁻ ions; weak bases (e.g., NH₄OH) produce fewer.
(d) Dilution: Mixing acid/base with water is exothermic, reduces H₃O⁺/OH⁻ concentration per unit volume.
- Caution: Add acid to water slowly with stirring to avoid splashing or container damage.
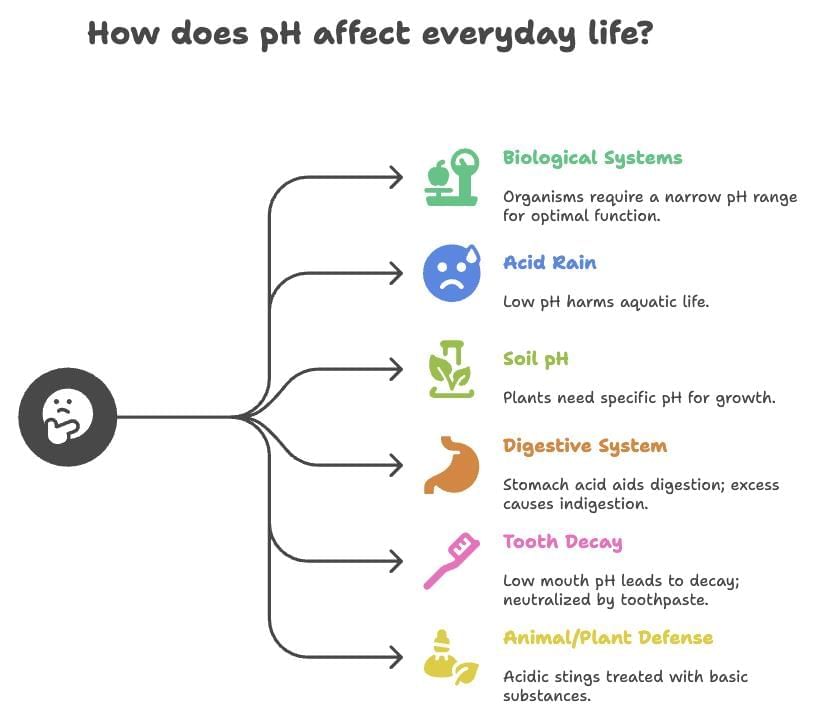
Importance of pH in Everyday Life
- Biological Systems: Organisms function in a narrow pH range (e.g., human body: 7.0–7.8).
- Acid Rain: pH < 5.6, harms aquatic life by lowering river water pH.
- Soil pH: Plants require specific pH for growth; tested using universal indicator paper.
- Digestive System: Stomach produces HCl for digestion; excess acid causes indigestion, treated with antacids (e.g., Mg(OH)₂).
- Tooth Decay: Occurs when mouth pH < 5.5; prevented by basic toothpaste neutralizing acid from bacterial sugar degradation.
- Animal/Plant Defense: Bee stings (methanoic acid, acidic) treated with baking soda (base); nettle stings (methanoic acid) treated with dock plant leaves (basic).
Salts
1. Family of Salts
Definition: Salts with same positive or negative ions belong to a family.
- Examples: NaCl, Na₂SO₄ (sodium salts); NaCl, KCl (chloride salts).
- Common Salts: K₂SO₄, Na₂SO₄, CaSO₄, MgSO₄, CuSO₄, NaCl, NaNO₃, Na₂CO₃, NH₄Cl.
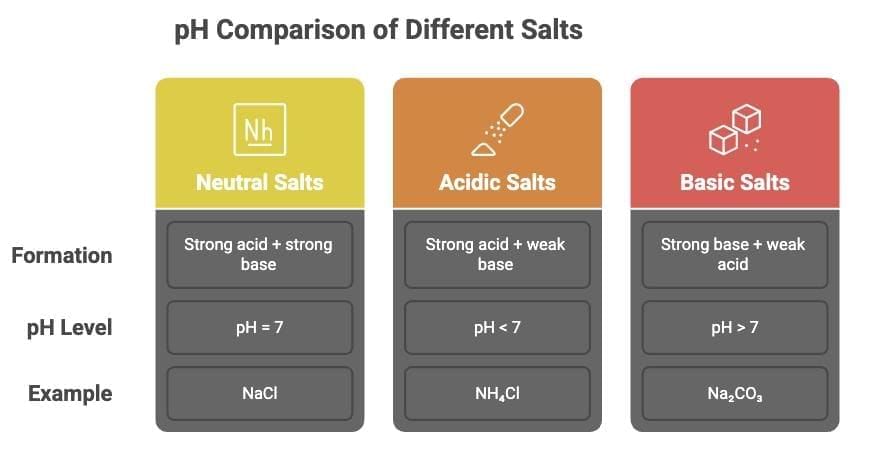
2. pH of Salts
- Neutral Salts: Formed from strong acid + strong base (pH = 7, e.g., NaCl).
- Acidic Salts: Strong acid + weak base (pH < 7, e.g., NH₄Cl).
- Basic Salts: Strong base + weak acid (pH > 7, e.g., Na₂CO₃).
- Testing: Check pH using universal indicator paper.
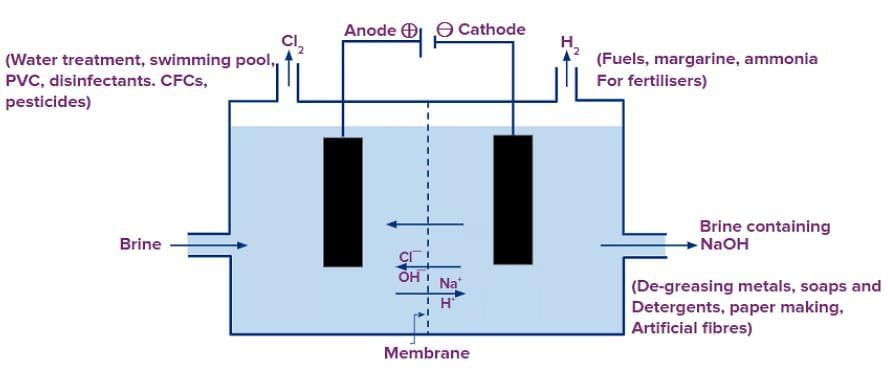
Sodium Hydroxide (NaOH)
Process: Chlor-alkali process via electrolysis of aqueous sodium chloride (brine).
Reaction:
2NaCl(aq) + 2H₂O(l) → 2NaOH(aq) + Cl₂(g) + H₂(g)Electrolysis Products:
Anode: Chlorine gas (Cl₂) released.
Cathode: Hydrogen gas (H₂) released.
Solution: Sodium hydroxide (NaOH) remains.
Bleaching Powder (Ca(OCl)₂ or CaOCl₂)
Preparation: Reaction of chlorine gas with dry slaked lime.
Ca(OH)₂(aq) + Cl₂(g) → CaOCl₂(aq) + H₂O(l)Uses:
Bleaching cotton and linen in the textile industry.
Oxidizing agent in various industries.
Disinfectant for potable water by eliminating microorganisms.
Baking Soda (Sodium Bicarbonate, NaHCO₃)
Preparation:
NaCl + H₂O + CO₂ + NH₃ → NH₄Cl + NaHCO₃Reaction on Heating:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂Uses:
Component in baking powder (with tartaric acid), producing CO₂ to make bread/cake rise:
NaHCO₃ + H⁺ → CO₂ + H₂O + Sodium salt of acidNeutralizes stomach acid as an antacid.
Used in soda-acid fire extinguishers.
Washing Soda (Sodium Carbonate, Na₂CO₃·10H₂O)
Preparation: Heating baking soda to form sodium carbonate, then recrystallization.
Na₂CO₃ + H₂O → Na₂CO₃·10H₂OUses:
Used in glass, soap, and paper industries.
Production of sodium compounds like borax.
Cleaning agent for household use.
Removes permanent water hardness.
Water of Crystallization: Contains 10 water molecules per formula unit, making it hydrated.
Water of Crystallization
Definition: Water molecules bound within a salt’s crystal structure.
 Salt Crystals
Salt CrystalsExamples:
Copper Sulfate (CuSO₄·5H₂O): Appears dry but contains water of crystallization. Heating removes water, turning it white; moistening restores blue color.
Washing Soda (Na₂CO₃·10H₂O): Hydrated with 10 water molecules.
Gypsum (CaSO₄·2H₂O): Contains 2 water molecules per formula unit.
Crystallization: Formation of solid crystals with organized atomic/molecular structure via precipitation, freezing, or gas deposition.
Examples of Crystals: Table salt (NaCl), sucrose (sugar), snowflakes, diamond, quartz.
Plaster of Paris (CaSO₄·½H₂O)
Preparation: Heating gypsum to 100°C (373K).
CaSO₄·2H₂O(s) → CaSO₄·½H₂O + 3/2H₂OChemical Note: Two CaSO₄ units share one water molecule.
Uses:
Medical Casting: Forms sturdy casts for broken bones when mixed with water.
 Use of Plaster of Paris
Use of Plaster of ParisSculpting and Art: Moldable for casts, masks, and decorative designs.
Home Decoration: Used for wall accents, ceiling tiles, figurines, and crafts.
|
80 videos|661 docs|80 tests
|
FAQs on Cheat Sheet: Acids, Bases and Salts - Science Class 10
| 1. What are the common properties of acids and bases? |  |
| 2. How is the strength of acids and bases determined? |  |
| 3. Why is pH important in everyday life? |  |
| 4. What are salts and how are they formed? |  |
| 5. What is the significance of the pH scale? |  |
















