Cheat Sheet: Metals and Non-metals | Science Class 10 PDF Download
| Table of contents |

|
| Physical Properties of Metals and Non-Metals |

|
| Chemical Properties of Metals and Non-Metals |

|
| Ionic Compounds |

|
| Occurrence of Metals |

|
| Extraction of Metals |

|
| Corrosion |

|
| Alloys |

|
Physical Properties of Metals and Non-Metals
1. Metals
Appearance: Shiny, lustrous surface (e.g., iron, copper, aluminium, magnesium).
Hardness: Generally hard, varies by metal (e.g., iron, zinc, lead, copper).
Malleability: Can be beaten into thin sheets (e.g., gold, silver most malleable).
Ductility: Can be drawn into wires (e.g., gold most ductile, 2 km wire from 1 g).
Conductivity:
Heat: Good conductors (e.g., silver, copper best; lead, mercury poor).
Electricity: Good conductors (e.g., copper in wires).
Sonority: Produce sound when struck (e.g., used in school bells).
Melting Point: High, except mercury (liquid), gallium, caesium (melt on palm).
Exceptions:
Alkali metals (Li, Na, K): Soft, low density, low melting points.
Mercury: Liquid at room temperature.
2. Non-metals
Appearance: Dull, non-lustrous (except iodine, which is lustrous).
Physical State: Solids (e.g., carbon, sulphur), gases (e.g., oxygen, hydrogen), or liquid (bromine).
Hardness: Generally brittle or soft (e.g., sulphur, iodine).
Malleability/Ductility: Non-malleable, non-ductile; break when hammered or stretched.
Conductivity: Poor conductors of heat and electricity (except graphite, which conducts electricity).
Sonority: Non-sonorous, no ringing sound.
Allotropes: Carbon has diamond (hardest, high melting point) and graphite (conducts electricity).
Chemical Properties of Metals and Non-Metals
1. Metal - Reaction with Oxygen
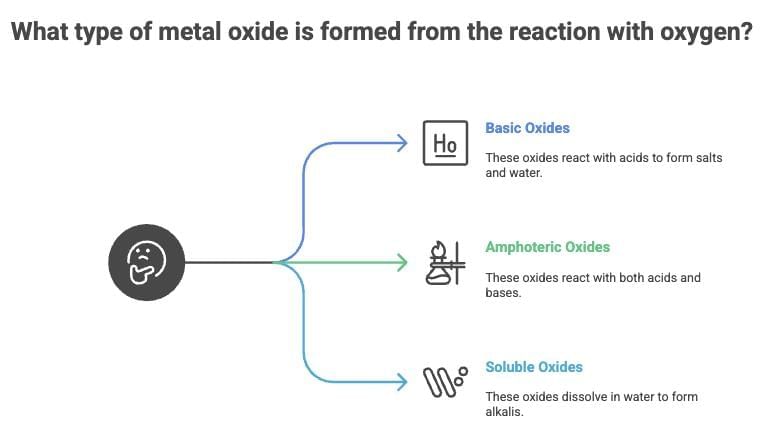
(a) Form metal oxides (basic or amphoteric).
Examples: 2Cu + O₂ → 2CuO (black); 4Al + 3O₂ → 2Al₂O₃
(b) Amphoteric Oxides: Al₂O₃, ZnO react with acids and bases.
Al₂O₃ + 6HCl → 2AlCl₃ + 3H₂O
Al₂O₃ + 2NaOH → 2NaAlO₂ + H₂O (sodium aluminate)
(c) Soluble Oxides: Na₂O, K₂O form alkalis in water.
Na₂O + H₂O → 2NaOH; K₂O + H₂O → 2KOH
(d) Reactivity: K, Na burn vigorously (stored in kerosene)due to their high reactivity; Mg, Al, Zn, Pb form oxide layers due to the moderate reactivity; Fe rusts; Cu forms CuO due to its low reactivity ; Ag, Au unreactive .
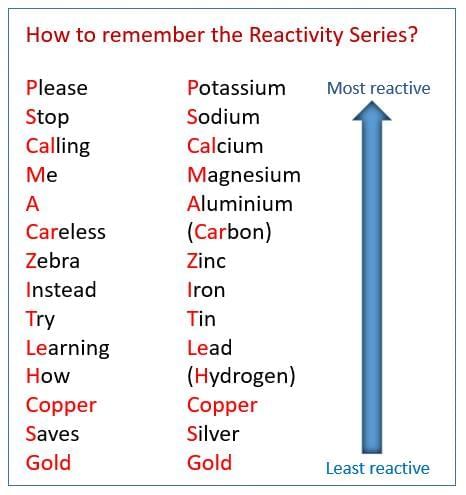
Reactivity Series: K > Na > Ca > Mg > Al > C > Zn > Fe > Sn > Pb > H > Cu > Ag > Au.
2. Metals - Reaction with Water
(a) Form metal oxide/hydroxide and hydrogen gas.
Metal + Water → Metal oxide/hydroxide + H₂
(b) High Reactivity: K, Na react violently with cold water, ignite H₂.
2K + 2H₂O → 2KOH + H₂ + heat
2Na + 2H₂O → 2NaOH + H₂ + heat
(c) Moderate Reactivity: Ca reacts with cold water; Mg with hot water; both float due to H₂ bubbles.
Ca + 2H₂O → Ca(OH)₂ + H₂
Mg + 2H₂O → Mg(OH)₂ + H₂
(d) Low Reactivity: Al, Fe, Zn react with steam; Pb, Cu, Ag, Au unreactive.
2Al + 3H₂O(g) → Al₂O₃ + 3H₂
3Fe + 4H₂O(g) → Fe₃O₄ + 4H₂
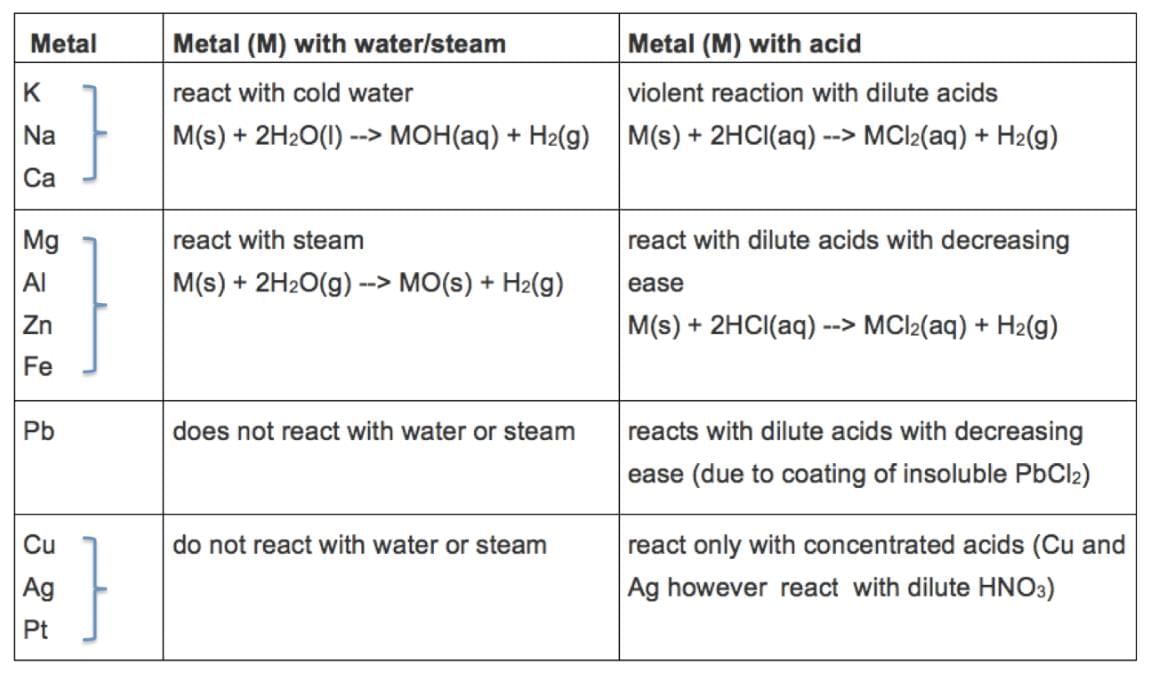
3. Metals - Reaction with Acids
(a) Form salt and hydrogen gas (except HNO₃, which oxidizes H₂).
Metal + Dilute acid → Salt + H₂
Examples: Mg + 2HCl → MgCl₂ + H₂; Zn + H₂SO₄ → ZnSO₄ + H₂
(b) Reactivity: Mg > Al > Zn > Fe (fastest bubble formation, highest temperature); Cu unreactive.
(c) Exception: Mg, Mn react with dilute HNO₃ to produce H₂.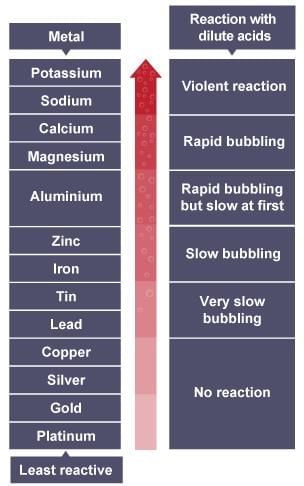
(d) Reaction with Metal Salts
A more reactive metal displaces less reactive metal from its salt solution.
Metal A + Salt of B → Salt of A + Metal B
Example: Fe + CuSO₄ → FeSO₄ + Cu (Cu displaced in CuSO₄ solution).
4. Non-Metal - Reaction with Oxygen
Form acidic or neutral oxides.
S + O₂ → SO₂ (acidic); SO₂ + H₂O → H₂SO₃(acid)
C + O₂ → CO₂ (acidic); CO₂ + H₂O → H₂CO₃ (acid)
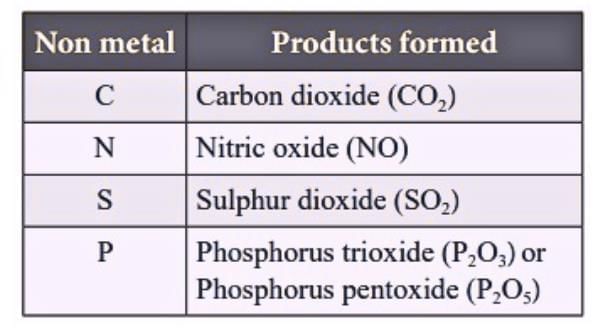 Table showing the oxides formed when different non-metals react with oxygen
Table showing the oxides formed when different non-metals react with oxygen
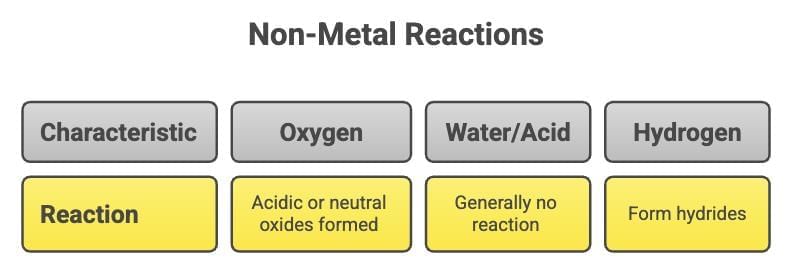
5. Non-Metal - Reaction with Water or Acid
- Non-metals generally do not react with water or acids.
6. Non-Metal - Reaction with Oxygen
- Form hydrides with hydrogen (e.g., H₂ + S → H₂S).
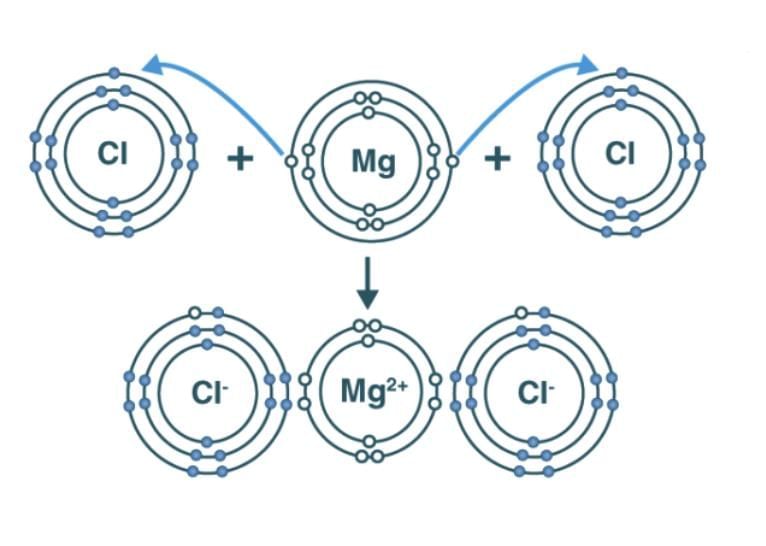
Ionic Compounds
Formation: Transfer of electrons from metal to non-metal.
Na → Na⁺ + e⁻; Cl + e⁻ → Cl⁻; forms NaCl.
Mg → Mg²⁺ + 2e⁻; 2Cl + 2e⁻ → 2Cl⁻; forms MgCl₂.
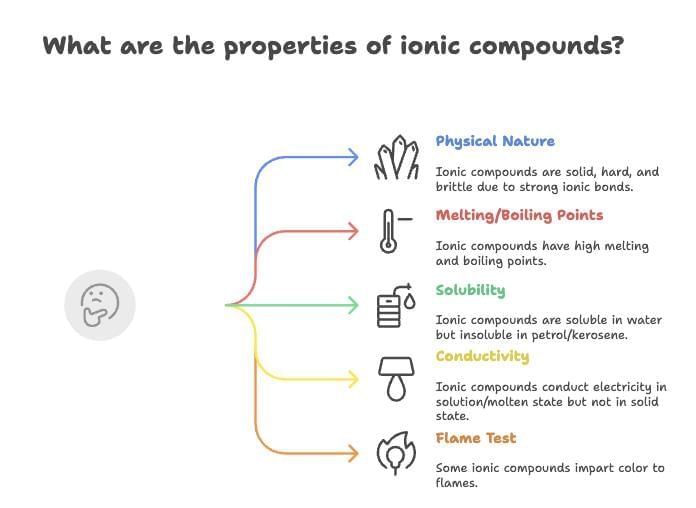
Properties of Ionic Compounds
Physical Nature: Solid, hard, brittle due to strong ionic bonds.
Melting/Boiling Points: High (e.g., NaCl: 1074 K melting, 1686 K boiling).
Solubility: Soluble in water, insoluble in petrol/kerosene.
Conductivity: Conduct electricity in solution/molten state due to mobile ions; non-conductive in solid state.
Flame Test: Some impart color (e.g., NaCl: yellow flame).
Occurrence of Metals
Source: Earth’s crust (minerals); seawater (e.g., NaCl, MgCl₂).
Minerals: Naturally occurring compounds; ores contain high metal content for profitable extraction.
Forms: Low reactivity metals (Ag, Au, Pt, Cu) found free or as sulphides/oxides; high reactivity metals (K, Na, Ca, Mg, Al) as compounds; medium reactivity metals (Zn, Fe, Pb) as oxides, sulphides, carbonates.
Extraction of Metals
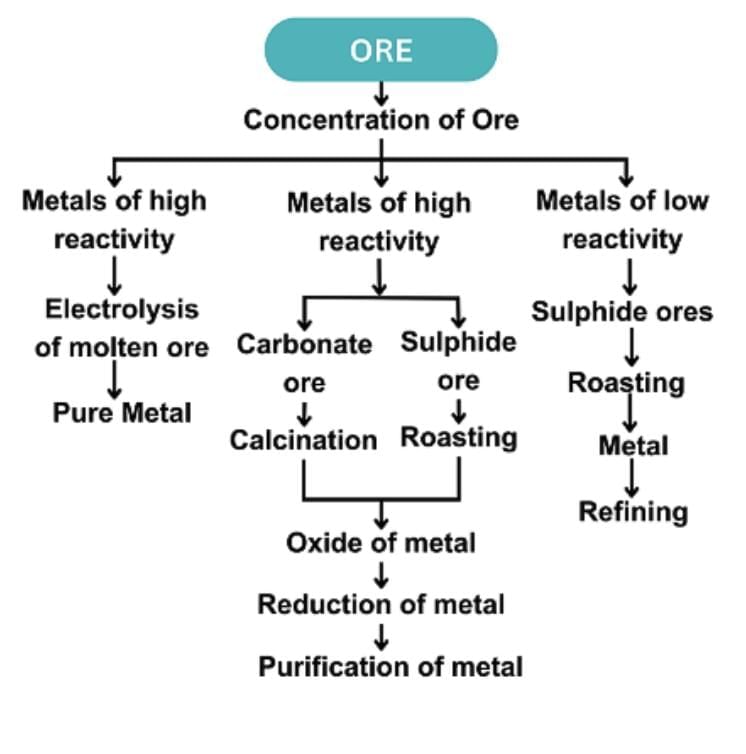
1. Low Reactivity (e.g., Hg, Cu):
Heating alone reduces oxides.
2HgS + 3O₂ → 2HgO + 2SO₂; 2HgO → 2Hg + O₂
2Cu₂S + 3O₂ → 2Cu₂O + 2SO₂; 2Cu₂O + Cu₂S → 6Cu + SO₂
2. Medium Reactivity (e.g., Zn, Fe, Pb):
Roasting: Sulphides to oxides (2ZnS + 3O₂ → 2ZnO + 2SO₂).
Calcination: Carbonates to oxides (ZnCO₃ → ZnO + CO₂).
Reduction: Carbon reduces oxides (ZnO + C → Zn + CO).
Displacement: Al reduces MnO₂ (3MnO₂ + 4Al → 3Mn + 2Al₂O₃).
Thermit Reaction: Fe₂O₃ + 2Al → 2Fe + Al₂O₃ (for railway tracks).
3. High Reactivity (e.g., Na, Mg, Al):
Electrolytic reduction of molten chlorides/oxides.
NaCl → Na⁺ + Cl⁻; Cathode: Na⁺ + e⁻ → Na; Anode: 2Cl⁻ → Cl₂ + 2e⁻
Al₂O₃ → Al at cathode, O₂ at anode.
4. Refining:
Electrolytic refining (e.g., Cu).
Anode: Impure Cu; Cathode: Pure Cu strip; Electrolyte: Acidified CuSO₄.
Pure Cu deposits on cathode; impurities form anode mud.
Corrosion
Definition: Deterioration of metals by air, moisture, or chemicals.
Silver: Forms black Ag₂S with H₂S in air.
Copper: Forms green basic copper carbonate with moist CO₂.
Iron: Forms rust (Fe₂O₃·nH₂O) with air and water (Activity 3.14).
Conditions for Rusting
Requires air and water (no rust in boiled water or dry air).
Prevention:
Painting, oiling, greasing, galvanising (Zn coating), chrome plating, anodising.
Alloying: Stainless steel (Fe + Ni + Cr) resists rust.
Galvanisation: Zn protects steel even if coating breaks (sacrificial protection).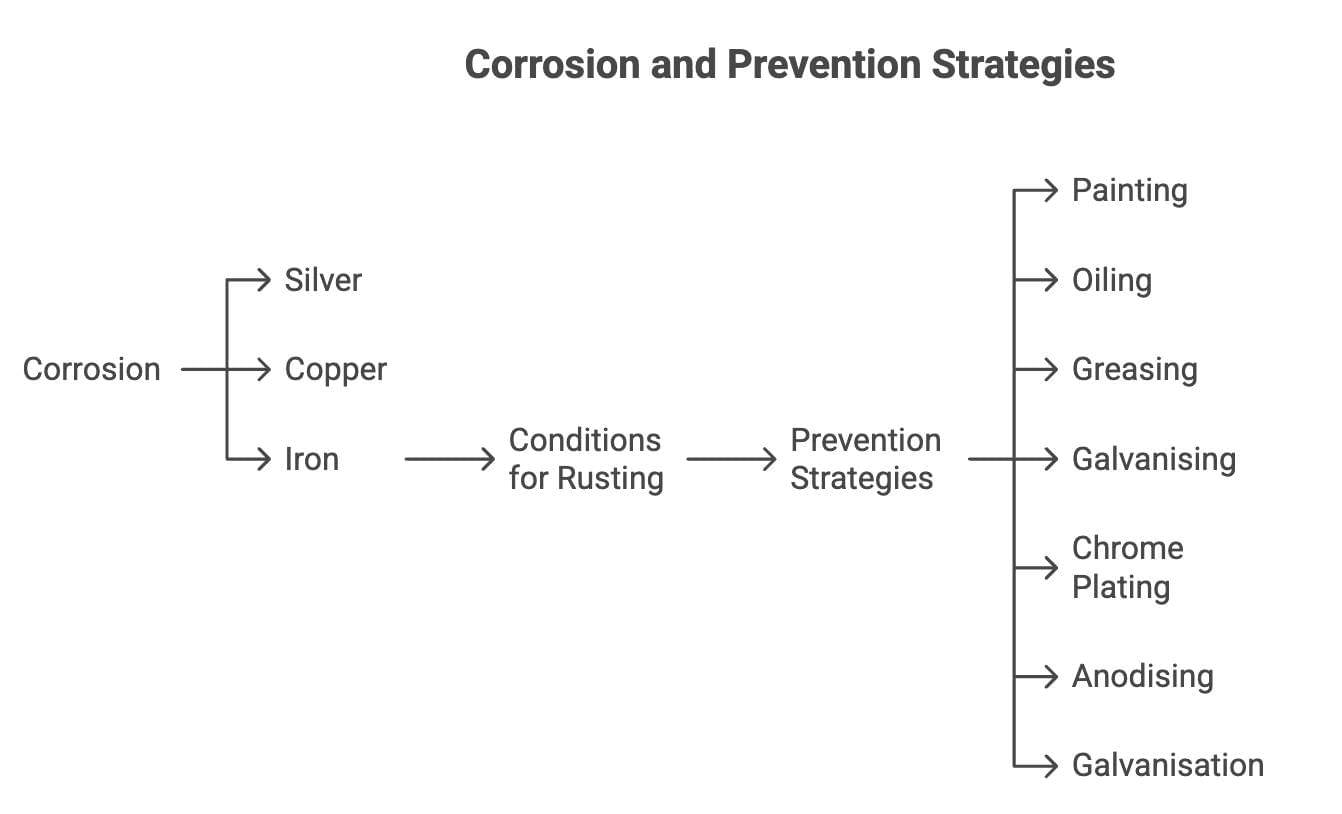
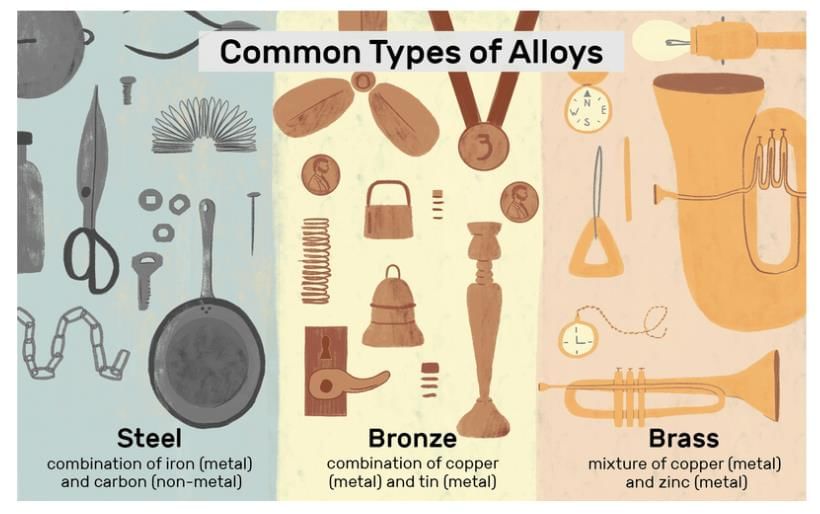
Alloys
Definition: Homogeneous mixture of metals or metal with non-metal.
Examples: Brass (Cu + Zn), Bronze (Cu + Sn), Stainless steel (Fe + Ni + Cr), Solder (Pb + Sn).
Properties: Lower conductivity, melting point than pure metals; enhanced strength, corrosion resistance.
Example: 22-carat gold (22 parts Au + 2 parts Cu/Ag) for jewellery; pure gold (24-carat) too soft.
Amalgams: Alloys with mercury.
|
80 videos|569 docs|80 tests
|
FAQs on Cheat Sheet: Metals and Non-metals - Science Class 10
| 1. What are the physical properties of metals and non-metals? |  |
| 2. How do ionic compounds differ from covalent compounds? |  |
| 3. What is the process of extraction of metals from their ores? |  |
| 4. What causes corrosion in metals, and how can it be prevented? |  |
| 5. What are alloys, and why are they important? |  |