Sure Shot Questions for Board Exams: Acids, Bases & Salts | Science Class 10 PDF Download
Introduction
The CBSE Class 10 chapter on Acids, Bases, and Salts is a cornerstone of the Chemistry syllabus, emphasizing concepts like pH, indicators, salts, and their real-world applications. Based on the analysis of previous year question papers (2015–2025), we’ve identified recurring themes and high-weightage topics that dominate the exams. These trends, combined with their significance in the syllabus, have guided our compilation of key questions and concepts most likely to reappear. Our predictions are rooted in the frequency of topics like pH scales, water of crystallization, and industrial processes, as well as CBSE’s consistent question patterns.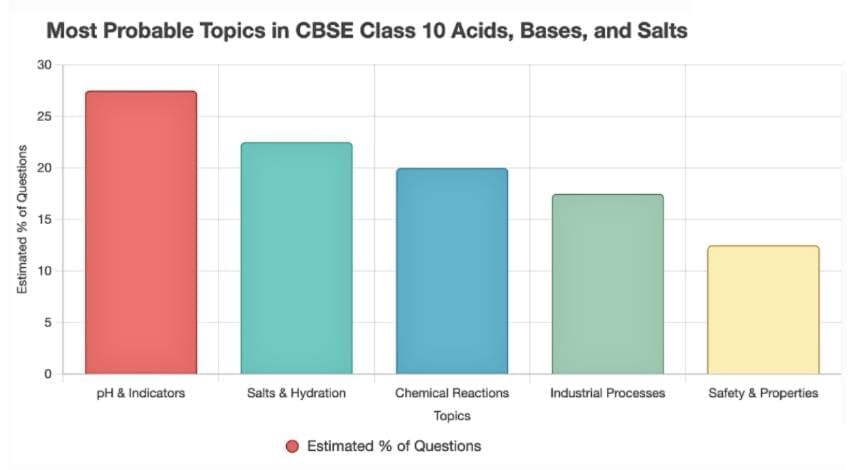
Below is a focused list of critical topics and repeated question types, supported by examples from past papers to help you prepare effectively for the upcoming exam.
Key Questions
Q1: The human body functions normally within the pH range of:
(a) 6.1 to 6.8
(b) 6.5 to 7.3
(c) 7.0 to 7.8
(d) 7.5 to 8.1
 View Answer
View Answer 
Ans: (c) 7.0 to 7.8
Blood pH is tightly regulated between 7.35–7.45 for normal body function.
Option (c) (7.0–7.8) is the closest range.
Q2: In one formula unit of salt 'X', seven molecules of water of crystallisation are present. The salt 'X' is:
(a) CuSO₄
(b) Na₂CO₃
(c) FeSO₄
(d) CaSO₄
 View Answer
View Answer 
Ans: (c) FeSO₄
FeSO₄·7H₂O (ferrous sulphate heptahydrate) has 7 water molecules.
CuSO₄·5H₂O has 5 water molecules.
Na₂CO₃·10H₂O has 10 water molecules.
CaSO₄·2H₂O (gypsum) has 2 water molecules.
Q3: Granulated zinc is added to sodium hydroxide solution and warmed. The product formed is:
(a) Na₂ZnO
(b) NaZnO₂
(c) Na₂ZnO₂
(d) NaZn(OH)₂
 View Answer
View Answer 
Ans: (c) Na₂ZnO₂
Zinc reacts with NaOH to form sodium zincate (Na₂ZnO₂) and hydrogen:
Zn + 2NaOH → Na₂ZnO₂ + H₂.
Zinc is amphoteric, reacting with bases to form zincate ions.
Q3: (a) A few crystals of ferrous sulphate were taken in a dry boiling tube and heated. Tiny water droplets were observed in the tube after some time.
(i) From where did these water droplets appear? Explain.
(ii) What color change will be observed during heating?
(iii) How many molecules of water are attached per molecule of FeSO4 crystal? Write the molecular formula of crystalline forms of (I) Copper sulphate, and (II) Sodium carbonate.
(iv) State how is Plaster of Paris obtained from gypsum. Write two uses of Plaster of Paris.
OR
(b) An acid ‘X’ present in tamarind when mixed with ‘Y’, produces a mixture ‘Z’. ‘Z’ on addition to a dough when heated makes cakes soft and spongy. ‘Y’ is prepared from common salt and helps in faster cooking.
(i) Write the common names of ‘X’, ‘Y’ and ‘Z’, and the chemical formula of ‘Y’.
(ii) How is ‘Y’ prepared and how does it help in making cakes soft and spongy? Illustrate the reaction with a suitable chemical equation.
(iii) Write the name and chemical formula of a mild base other than ‘Y’ used as an antacid.
 View Answer
View Answer 
Ans: (a) (i)The water droplets appear due to the evaporation of water of crystallisation in ferrous sulphate crystals when heated.
(ii) The colour change observed during heating is from green to white.
(iii) Each molecule of FeSO4 crystal has seven water molecules attached, represented as FeSO4 · 7H2O. The molecular formulas for the crystalline forms are:
- (I) Copper sulphate: CuSO4 · 5H2O
- (II) Sodium carbonate: Na2CO3 · 10H2O
(iv) Plaster of Paris is obtained by heating gypsum (CaSO4 · 2H2O) at 373 K, which causes it to lose water molecules.
Two uses of Plaster of Paris are:
- It is used by doctors to support fractured bones.
- It is also used in making decorative items.
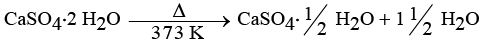
OR
(b) (i) X-Tartaric acid , Y-Baking soda , Z- Baking powder Y- NaHCO3
(ii) NaCl + H2O +CO2 +NH3 → NH4Cl +NaHCO3
NaHCO3 + H+ → CO2 + H2O + Sodium salt of acid CO2 released during heating makes the cake soft and spongy.
(iii) Magnesium hydroxide; Mg(OH)2
Q4: On heating X at 373 K, it loses water molecules and becomes Y. Y is a substance which doctors use for supporting fractured bones in the right position.
(i) Identify X and V.
(ii) How can X be reobtained from Y?
 View Answer
View Answer 
Ans: (i) X is gypsum (CaSO4 · 2H2O) and Y is Plaster of Paris (CaSO4 · 0.5H2O). V is the substance doctors use for supporting fractured bones in the right position.
(ii) X can be reobtained from Y by:
- Adding water to Plaster of Paris.
- This process converts it back to gypsum.
The reaction is: CaSO4 · 0.5H2O + 1.5H2O → CaSO4 · 2H2O
Q5: The industrial process used for the manufacture of caustic soda involves electrolysis of an aqueous solution of compound 'X'. In this process, two gases Y and Z are liberated. Y is liberated at the cathode and Z, which is liberated at the anode, on treatment with dry slaked lime, forms a compound 'B'. Name X, Y, Z, and B.
 View Answer
View Answer 
Ans: Compound X: Sodium chloride (NaCl).
- Gas Y: Hydrogen gas (H2) is liberated at the cathode.
- Gas Z: Chlorine gas (Cl2) is liberated at the anode.
- Compound B: Bleaching powder (CaOCl2) is formed when chlorine gas reacts with dry slaked lime (Ca(OH)2).
Q6: During electrolysis of brine, a gas 'G' is liberated at the anode. When this gas 'G' is passed through slaked lime, a compound 'C' is formed, which is used for disinfecting drinking water.
(i) Write the formula of 'G' and 'C'.
(ii) State the chemical equations involved.
(iii) What is the common name of compound 'C'? Give its chemical name.
 View Answer
View Answer 
Ans: (i) The formula of 'G' is Cl2 and the formula of 'C' is CaOCl2.
(ii) The chemical equations involved are:
- At the anode during electrolysis of brine:
2NaCl(aq) + 2H2O(l) → Cl2(g) + H2(g) + 2NaOH(aq) - When chlorine gas (G) is passed through slaked lime:
Ca(OH)2(s) + Cl2(g) → CaOCl2(s) + H2O(l)
(iii) The common name of compound 'C' is bleaching powder. Its chemical name is calcium hypochlorite.
Q7: (a) You are provided with concentrated sulphuric acid. Describe the process of preparing a dilute solution of sulphuric acid.
(b) What is the effect of dilution on (H3O+/OH-) ratio?
(c) If the H3O+ ion concentration is increased in a solution, will the pH increase or decrease? What are the probable colours of pH paper if the pH range is 0-5 ?
 View Answer
View Answer 
Ans: (a) To prepare a dilute solution of sulphuric acid, follow these steps:
- Start with a beaker filled with a sufficient amount of water.
- Slowly add the concentrated acid to the water while stirring continuously.
- Always add acid to water, never the other way around, to minimise the risk of splashing or glass breakage due to heat.
(b) The effect of dilution on the (H₃O⁺/OH⁻) ratio:
- When an acid or base is diluted, the concentration of ions (H₃O⁺ and OH⁻) per unit volume decreases.
- This results in a reduced (H₃O⁺/OH⁻) ratio.
(c) If the concentration of H₃O⁺ ions increases in a solution:
- The pH will decrease.
- For pH paper in the range of 0-5, the probable colours will be red or orange, indicating acidic conditions.
Q8: The salt present in tooth enamel is:
(a) Calcium phosphate
(b) Magnesium phosphate
(c) Sodium phosphate
(d) Aluminium phosphate
 View Answer
View Answer 
Ans: (a)
- Tooth enamel is primarily composed of a mineral known as hydroxyapatite, which is a form of calcium phosphate.
- This mineral plays a crucial role in: Strengthening teeth and making them resistant to decay
- Thus, the correct answer is (a) Calcium phosphate.
Q9: Tooth enamel is made up of calcium hydroxyapatite (a crystalline form of calcium phosphate). This chemical starts corroding in the mouth when the pH is:
(a) 7
(b) 5
(c) 10
(d) 14
 View Answer
View Answer 
Ans: (b) 5
Tooth enamel (Ca₅(PO₄)₃OH) corrodes in acidic conditions.
pH below 5.5 causes demineralization due to acid attack.
pH 7 is neutral, 10 and 14 are basic, not corrosive to enamel.
Q10: The name of the salt used to remove permanent hardness of water is
(a) Sodium hydrogen carbonate (NaHCO3)
(b) Sodium chloride (NaCI)
(c) Sodium carbonate decahydrate (Na2CO3.10H2O)
(d) Calcium sulphate hemihydrate (CaSO4. 1/2 H2O)
 View Answer
View Answer 
Ans: (c)
Sol: Na2CO3.10H2O is used to remove permanent hardness of water. Washing soda (Na2CO3.10H2O) reacts with soluble calcium and magnesium chlorides and sulphates in hard water to form insoluble carbonates, that can be removed by filtration and then water becomes soft.
Q11: (i) Suggest a safe procedure for diluting a strong concentrated acid.
(ii) Name the salt formed when sulphuric acid is added to sodium hydroxide and write its pH.
(iii) Dry hydrochloric acid (HCl) gas does not change the colour of dry blue litmus paper. Why?
 View Answer
View Answer 
Ans: (i)
- During the dilution of a strong concentrated acid, always add acid to water and not the water to acid.
- The dissociation of an acid in water is a highly exothermic process, as the acid has a strong affinity for water.
- Adding water to acid can cause a violent reaction due to the rapid generation of heat.
- By adding acid to water slowly and with constant stirring, the heat generated can be dissipated more effectively, ensuring a safe dilution process.
(iii) Dry hydrochloric acid (HCl) gas does not change the colour of dry blue litmus paper because:
- The colour change requires the presence of hydrogen ions (H+).
- HCl gas can only produce these ions in an aqueous solution, where it dissociates.
- Without water, HCl gas does not release H ions, hence no colour change occurs.
Q12: Sodium hydroxide is termed as alkali while ferric hydroxide is not because:
(a) Sodium hydroxide is a strong base, while ferric hydroxide is a weak base.
(b) Sodium hydroxide is a base which is soluble in water while ferric hydroxide is also a base but it is not soluble in water.
(c) Sodium hydroxide is a strong base while ferric hydroxide is a strong acid.
(d) Sodium hydroxide and ferric hydroxide both are strong base but the solubility of sodium hydroxide in water is comparatively higher than that of ferric hydroxide.
 View Answer
View Answer 
Ans: (b)
Sol: Sodium hydroxide is classified as an alkali, while ferric hydroxide is not due to the following reasons:
- Sodium hydroxide is a strong base, whereas ferric hydroxide is a weak base.
- Sodium hydroxide is soluble in water, while ferric hydroxide is not.
- Sodium hydroxide is not a strong acid; it is a base, while ferric hydroxide does not fit this classification.
- Both are bases, but sodium hydroxide has a higher solubility in water compared to ferric hydroxide.
Q13: Two solutions M and N give red and blue colour respectively with a universal indicator.
(i) In which solution will the hydrogen ion concentration be more? Justify your answer.
(ii) If both M and N solutions are mixed and the resultant mixture is tested with a universal indicator, it turns green. What is the nature of the salt formed? Justify your answer.
 View Answer
View Answer 
Ans: (i) Solution M gives a red color with a universal indicator, indicating that it is an acidic solution with a higher hydrogen ion concentration. Solution N gives a blue color with a universal indicator, indicating that it is a basic solution with a lower hydrogen ion concentration.
(ii) When solutions M and N are mixed, the resulting green color with a universal indicator indicates that the mixture is neutral. This suggests that a salt solution is formed, which is neither acidic nor basic.
Q14: Consider the following salts:
(i) yCI
(ii) NH4X
(iii) ZCO3
(a) What would be the pH of the salt solution if in yCI, y is sodium? Give a reason for your answer.
(b) If in salt NH4X, X is nitrate, then its solution will give what colour with a universal indicator? Why?
(c) What would be the change in colour in a blue litmus solution if ZCO3 is added to it and Z is potassium?
 View Answer
View Answer 
Ans: (a) If in yCI, y is sodium, the salt formed is NaCI. NaCI is a salt of a strong acid (HCI) and a strong base (NaOH), making it a neutral salt. Hence, the pH of the salt solution would be 7.
(b) If in salt NH4X, X is nitrate, the salt formed is NH4NO3. NH4NO3 is a salt of a weak base (NH4OH) and a strong acid (HNO3). It is an acidic salt and will give an orange-yellow colour with a universal indicator.
(c) Potassium carbonate (K₂CO₃) is a basic salt formed from a strong base (KOH) and a weak acid (H₂CO₃). In solution, it hydrolyzes to produce OH⁻ ions, making the solution basic (pH > 7):
K₂CO₃ + H₂O ⇌ 2K⁺ + HCO₃⁻ + OH⁻
Since blue litmus paper remains blue in basic solutions and only turns red in acidic solutions, adding K₂CO₃ will not change its color—it stays blue.
Q13: The following questions are source-based/case-based questions. Read the case carefully and answer the questions that follow:
Three metal samples of magnesium, aluminium and iron were taken and rubbed with sand paper. These samples were then put separately in test tubes containing dilute hydrochloric acid. Thermometers were also suspended in each test tube so that their bulbs dipped in the acid. The rate of formation of bubbles was observed. The above activity was repeated with dilute nitric acid and the observations were recorded.
Answer the following questions:
(a) When activity was done with dilute hydrochloric acid, then in which one of the test tubes was the rate of formation of bubbles the fastest and the thermometer showed the highest temperature?
(b) Which metal did not react with dilute hydrochloric acid? Give reason.
(c) (i) Why is hydrogen gas not evolved when a metal reacts with dilute nitric acid? Name the ultimate products formed in the reaction.
OR
(c) (ii) Name the type of reaction on the basis of which reactivity of metals is decided. You have two metals X and Y. How would you decide which is more reactive than the other?
 View Answer
View Answer 
Ans: (a) The test tube with magnesium had the fastest rate of bubble formation and the highest temperature.
(b) All three metals react with dilute hydrochloric acid as they are more reactive than hydrogen.
(c)(i) Hydrogen gas is not produced when a metal reacts with dilute nitric acid because nitric acid is a strong oxidising agent. It oxidises the hydrogen gas to water. The ultimate products are water and nitrogen oxides.
OR
(c)(i) The type of reaction that determines the reactivity of metals is a displacement reaction. If metal X displaces metal Y from its salt solution, then metal X is more reactive than metal Y, and vice versa.
Important Topics for Preparation
1. pH Scale and Indicators
Concept: pH measures acidity (pH < 7), neutrality (pH = 7), or basicity (pH > 7). Indicators show pH via color changes.
Key Points:
1. pH Ranges: Strong acids (~1–3), weak acids (~4–6), neutral (~7), weak bases (~8–10), strong bases (~11–14).
2. Indicators:
- Litmus: Blue turns red in acids; red turns blue in bases.
- Phenolphthalein: Colorless in acids/neutral, pink in bases.
- Methyl Orange: Red in acids, yellow in bases.
- Universal Indicator: Shows pH range (red: acidic, green: neutral, blue/purple: basic).
- Olfactory Indicators: Onion, clove oil, vanilla (odor changes in acid/base).
- Natural Indicators: Turmeric (yellow in acid, red in base), red cabbage.
3. Applications: Tooth enamel corrosion (pH < 5.5), human body pH (7.0–7.8), acid rain effects (pH < 5.6 harms aquatic life).
Focus: Memorize indicator color changes and pH ranges. Practice arranging solutions by pH
2. Properties of Acids and Bases
Concept: Acids release H⁺/H₃O⁺ ions; bases release OH⁻ ions or accept H⁺.
Key Points:
1. Acids: Sour taste, turn blue litmus red, react with metals (H₂ gas), bases (salt + water), carbonates (CO₂).
- Strong acids (e.g., HCl, H₂SO₄, HNO₃) fully dissociate.
- Weak acids (e.g., CH₃COOH, tartaric acid) partially dissociate.
2. Bases: Bitter taste, slippery, turn red litmus blue.
- Strong bases (e.g., NaOH, KOH) fully dissociate.
- Weak bases (e.g., NH₄OH, Mg(OH)₂) partially dissociate.
3. Amphoteric Substances: Zn, Al, Al₂O₃ react with both acids (form H₂/salts) and bases (e.g., Na₂ZnO₂).
4. Dry vs. Wet Acids: Dry HCl gas doesn’t dissociate (no H⁺, no litmus change); wet HCl forms H₃O⁺ (acidic).
Focus: Learn reactions (e.g., Zn + HCl → ZnCl₂ + H₂; Zn + NaOH → Na₂ZnO₂ + H₂) and why dry HCl is neutral
3. Salts and Their Properties
Concept: Salts form from acid-base neutralization (e.g., HCl + NaOH → NaCl + H₂O).
Key Points:
1. Types:
- Neutral salts (strong acid + strong base, pH ~7, e.g., NaCl, KNO₃).
- Acidic salts (strong acid + weak base, pH < 7, e.g., NH₄Cl).
- Basic salts (weak acid + strong base, pH > 7, e.g., Na₂CO₃).
2. Family of Salts: Share same cation (e.g., NaCl, Na₂CO₃) or anion (e.g., K₂SO₄, Na₂SO₄).
3. pH Testing: Use universal indicator or litmus to determine salt solution pH
Focus: Identify salt types and predict pH based on parent acid/base strength.
4. Water of Crystallization
Concept: Fixed water molecules in a salt’s crystalline structure (e.g., CuSO₄·5H₂O).
Key Points:
1. Examples of water of crystallisation:
- FeSO₄·7H₂O (ferrous sulphate, green).
- CuSO₄·5H₂O (copper sulphate, blue).
- Na₂CO₃·10H₂O (washing soda).
- CaSO₄·2H₂O (gypsum).
- CaSO₄·½H₂O (plaster of Paris).
2. Heating Effects: Loss of water (droplets), color change (e.g., blue CuSO₄ → white; green FeSO₄ → brown Fe₂O₃).
3. Proof: Add water to anhydrous salt (e.g., white CuSO₄ → blue) to confirm water of crystallization.
Focus: Memorize formulas, water molecules, and heating observations
5. Chemical Reactions of Acids, Bases, and Salts
Concept: Predict products and balance equations for common reactions.
Key Points:
- Acid + Metal: H₂ gas (e.g., Zn + 2HCl → ZnCl₂ + H₂; test: pop sound).
- Acid + Base: Salt + water (e.g., HCl + NaOH → NaCl + H₂O).
- Acid + Carbonate: Salt + H₂O + CO₂ (e.g., CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂; test: lime water milky).
- Base + Non-metal Oxide: Salt + water (e.g., Ca(OH)₂ + CO₂ → CaCO₃ + H₂O).
- Amphoteric Reactions: Zn/Al with NaOH (e.g., Zn + 2NaOH → Na₂ZnO₂ + H₂).
Focus: Practice balancing equations and identifying products
6. Industrial Processes and Applications
Concept: Preparation and uses of key compounds.
Key Points:
1. Chlor-Alkali Process(electrolysis of brine):
- Products: NaOH (soap), Cl₂ (disinfectants/bleach), H₂ (ammonia).
- Equation: 2NaCl + 2H₂O → 2NaOH + Cl₂ + H₂.
2. Bleaching Powder(Ca(OCl)Cl):
- Preparation: Ca(OH)₂ + Cl₂ → Ca(OCl)Cl + H₂O.
- Uses: Disinfecting water, bleaching clothes.
3. Washing Soda(Na₂CO₃·10H₂O):
- Preparation: 2NaHCO₃ → Na₂CO₃ + H₂O + CO₂; then Na₂CO₃ + 10H₂O → Na₂CO₃·10H₂O.
- Uses: Laundry, water softening (removes Ca²⁺/Mg²⁺).
4. Baking Soda/Powder:
- Baking soda (NaHCO₃): Antacid, fire extinguisher, releases CO₂ in cooking.
- Baking powder: NaHCO₃ + acid (e.g., tartaric acid) for consistent CO₂ release.
- Reaction: NaHCO₃ + H⁺ → Na⁺ + H₂O + CO₂.
5. Plaster of Paris(CaSO₄·½H₂O):
- Preparation: CaSO₄·2H₂O → CaSO₄·½H₂O + 1½H₂O (heat at 373 K).
- Uses: Fractured bone support, decorative items.
Focus: Learn preparation reactions and two uses per compound.
7. Practical Safety and Observations
Concept: Safe handling and experimental observations.
Key Points:
1. Dilution: Add acid to water (not reverse) to avoid exothermic splashing
2. Gas Tests:
- H₂: Pop sound with burning match.
- CO₂: Turns lime water milky (Ca(OH)₂ → CaCO₃).
- HCl: No change on dry litmus; red on wet litmus.
3. Heating Salts: Color changes (CuSO₄: blue to white; FeSO₄: green to brown), water droplets, gas odors (e.g., SO₂/SO₃ from FeSO₄).
4. Antacids: Neutralize stomach acid (e.g., NaHCO₃ + HCl → NaCl + H₂O + CO₂).
Focus: Understand safety (dilution) and memorize observation-based questions.
8. Applications in Daily Life
Concept: Relate chemistry to real-world scenarios.
Key Points:
- Food: Tartaric acid in tamarind (turns litmus red), baking soda/powder for spongy cakes
- Health: Antacids (NaHCO₃, Mg(OH)₂) neutralize stomach acid; bee/nettle stings (formic acid, treat with base like NaHCO₃).
- Environment: Acid rain (pH < 5.6) harms aquatic life; soil pH adjustment with lime (CaO/CaCO₃).
- Industry: Na₂CO₃ in glass/soap, Cl₂ in disinfectants, CaSO₄·½H₂O in construction.
Focus: Connect chemical properties to uses.
Study Tips
- Memorize: Formulas (e.g., Na₂CO₃·10H₂O, CaSO₄·½H₂O), pH ranges, indicator colors, key reactions.
- Practice: Balance equations, predict products, and solve pH ordering questions.
- Focus on Repeated Questions: pH/indicators, water of crystallization, chlor-alkali, baking soda uses (see repeated questions list).
- Diagrams: Understand setups for HCl gas preparation, electrolysis of brine
- NCERT: Revise examples (e.g., CuSO₄ heating, lime water test) and activities.
These topics cover ~90% of questions from 2015–2025. For deeper focus, prioritize pH/indicators, salts, and industrial processes, as they dominate recent papers. If you need specific resources visit Acids, Bases and Salts on EduRev!
|
80 videos|569 docs|80 tests
|
FAQs on Sure Shot Questions for Board Exams: Acids, Bases & Salts - Science Class 10
| 1. What are acids, bases, and salts, and how are they classified? |  |
| 2. What is the pH scale, and how does it relate to acids and bases? |  |
| 3. How do indicators work, and what are some common examples used in acid-base chemistry? |  |
| 4. What is the neutralization reaction, and what are its products? |  |
| 5. What are some practical applications of acids, bases, and salts in everyday life? |  |
















