(ii) Similarly, piece of charcoal ,by holding it with tongs and placing it near a candle flame or Bunsen burner, that charcoal burns in the presence of air. This process is similar to how coal burns, producing carbon dioxide, heat, and light.
Combustion and Flame Class 8 Notes Science Chapter 4
| Table of contents |

|
| What is Combustion? |

|
| How Do We Control Fire? |

|
| Types of Combustion |

|
| Flame |

|
| Structure of flame |

|
| What is a Fuel? |

|
| Fuel efficiency |

|
Introduction
We use various fuels like wood, coal, petrol, and CNG at home, in industries, and for running vehicles. Have you ever notice the difference between how a candle burns with a flame while coal doesn't? In this chapter, we'll explore the chemical process of burning and the types of flames different fuels produce.For example:- (i) when magnesium ribbon burns, it produce magnesium oxide, along with heat and light.
 Burning of magnesium ribbon
Burning of magnesium ribbon
 Burning of charcoal
Burning of charcoal
What is Combustion?
The process in which a substance undergoes a chemical reaction in the presence of air (oxygen) to produce heat and light is called combustion.
The substance that undergoes combustion is said to be combustible substance .
Sometimes, light is also given off during combustion, either as a flame or as a glow.
In the reactions mentioned above magnesium and charcoal are combustible substances and the reaction type is combustion .
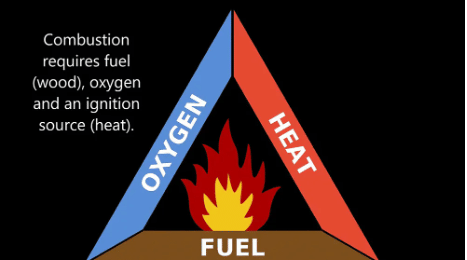
Combustion is a chemical process.
Examples:
- Burning of Wood or Coal to heat your home.
- Burning of Petrol or Diesel to run your Car.
- Combustion of Natural Gas or LPG to cook on your stove.
- For the production of energy in thermal power plants
- Fireworks
Combustible Substances
- Substances that go under combustion are known as combustible substances. Combustible substances are also known as fuel.
- The fuel may be in a solid, liquid or gaseous state.
Example: Wood, Charcoal, LPG, Kerosene, Petrol, Diesel, etc.

Non - Combustible substances
Substances that do not catch fire readily in the presence of air and do not produce heat and light are called non-combustible substance. Even if a non-combustible substance is exposed to flame, it will not burn. Examples – Glass, and stones.
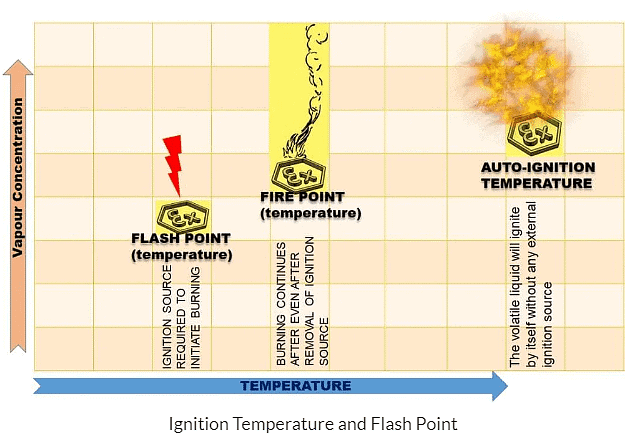
Ignition Temperature
- Ignition temperature, also known as the ignition point, is the minimum temperature at which a substance can start to burn and combust. This can happen to a substance in any state, whether it's solid, liquid, or gas.
- A substance won't burn unless it reaches its ignition temperature, such as phosphorus, which ignites at 35°C. If the air temperature is 35°C or higher, phosphorus can catch fire without additional heating.
- A matchstick can't burn by itself; it needs to reach a specific temperature, known as the ignition temperature, to catch fire.
- Different substances have different ignition temperatures. For example, kerosene oil ignites with a slight increase in temperature, while wood requires a much higher temperature.
- Inflammable substances: The substances that have very low ignition temperatures and can easily catch fire with a flame are known as inflammable substances, for example, diesel, LPG, and acetone. These have very low ignition temperatures and can easily catch fire.
Do you know?
- Early safety matches were made using a mixture of antimony trisulphide, potassium chlorate, and white phosphorus, along with glue and starch, on the match head. Striking the match against a rough surface would ignite the white phosphorus due to the frictional heat, starting the combustion. However, white phosphorus was hazardous for both manufacturers and users.
- Today’s safety matches have a head containing only antimony trisulphide and potassium chlorate. The striking surface includes powdered glass and a small amount of red phosphorus, which is less dangerous. When struck, red phosphorus is converted into white phosphorus, which then reacts with potassium chlorate to produce enough heat to ignite the antimony trisulphide and start the combustion.
Conditions for Combustion
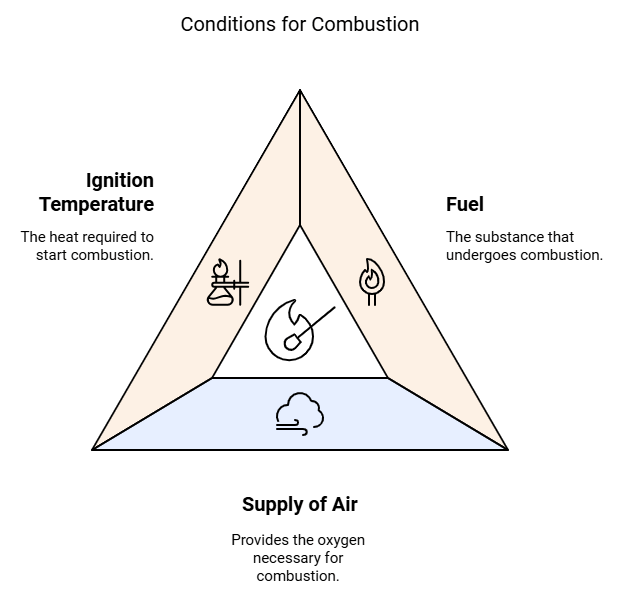
There are three conditions necessary for combustion:
- Fuel: Fuel is the substance that undergoes combustion.
- Supply of Air: Oxygen helps in combustion. Air contains about 21% of oxygen, thus the supply of air makes the oxygen available which helps in combustion. Without oxygen, combustion will not take place.
- Ignition Temperature: To burn a combustible substance, it must reach its ignition temperature. If a combustible substance does not reach or above its ignition temperature, it will not catch fire and combustion will not take place.
Thus, the above three conditions are necessary for combustion to take place. If any one of the three will not be available, combustion will not take place.
 Fire Triangle
Fire Triangle
How Do We Control Fire?
Generally, water is used to control fire. Water brings down the temperature of the combustible substance below its ignition temperature. The water vapor surrounds the combustible material, thus helping cut off the supply of air so that the fire is extinguished.
Fire produced by the burning of oil or petrol cannot be controlled by throwing water on it because water being heavier than oil and oil floating on water continues to burn.
We know that there are three conditions necessary for producing and sustaining combustion.
- Presence of a combustible substance.
- Presence of a supporter of combustion.
- Attainment of ignition or kindling temperature.
Thus, fire can be controlled by removing one or more of these fire control requirements.
Fire Brigade
- When a fire breaks out in homes, forests, or shops, quick action is crucial to prevent major damage and loss of life.
- Firefighters use extinguishers to control fires by eliminating one of the three essential conditions for combustion: fuel, air, or heat. Since the entire building acts as fuel, cutting off the fuel supply is difficult. Instead, firefighters focus on reducing air supply and lowering the temperature below the ignition point to put out the fire.
Fire Extinguisher
- Water is one of the best, cheapest and oldest fire extinguishers. By pouring water over the combustible material, the temperature can be cooled down. Cooling down brings the combustible materials below their ignition temperature.
- In addition to this, water vapour surrounds the combustible material which stops the supply of air. Removal of these two conditions puts off the fire. This is the cause that firemen generally pour water over the materials which have caught fire.
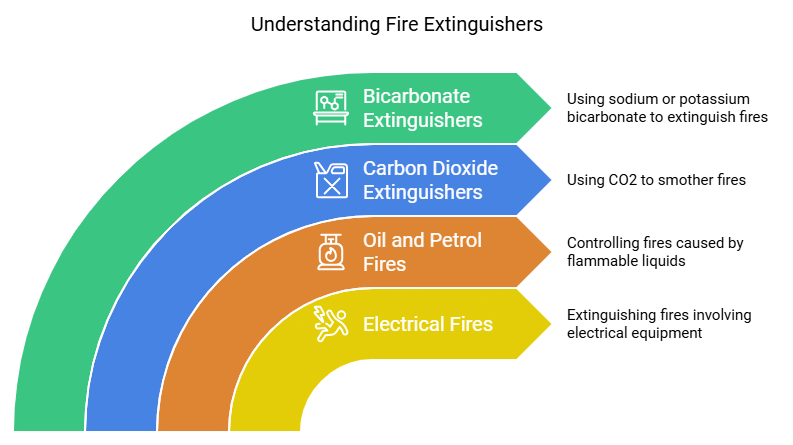
1. Controlling fire when electrical equipment is on fire
- Water is not suitable in the case when electrical equipment or oil catches fire.
- In the case of electrical equipment on fire, pouring water over them may prove disastrous because normal water is a good conductor of electricity.
- It may conduct electricity and can harm the people who are trying to control the fire.
2. Controlling fire in the case of oil, petrol, etc.
- Water is heavier than oil. So when water is poured over oil. It comes on top and keeps on burning. In such cases, a fire extinguisher is used.
3. Carbon dioxide as a fire extinguisher
- Carbon dioxide does not support combustion and hence is considered the best fire extinguisher.
- Carbon dioxide is heavier than air and hence covers the material which is burning. By covering the material, the supply of oxygen is stopped. This puts off further combustion and fire is controlled.
- Under high pressure, carbon dioxide liquefies and takes less space because of compression. Liquid carbon dioxide is stored in cylinders.
- A nozzle is attached to the cylinder to release carbon dioxide. When the nozzle is opened, carbon dioxide starts coming out from the cylinder because of high pressure. It expands and covers the combustible materials as a blanket.
- This cuts off the supply of oxygen to combustible materials. Because of expansion, the temperature of carbon dioxide decreases which decrease the temperature and brings down the combustible material below their ignition temperature. Thus, the stoppage of the supply of oxygen and bringing down the temperature below the ignition temperature of combustible materials put off the fire.
4. Sodium bicarbonate or potassium bicarbonate as a fire extinguisher
- Sodium bicarbonate (baking soda) or potassium bicarbonate releases carbon dioxide on heating.
- Thus, when powder of sodium bicarbonate or potassium bicarbonate is spread over or near the fire, they release carbon dioxide which covers the burning materials and cuts off the supply of oxygen to them. This puts off the fire.
Types of Combustion
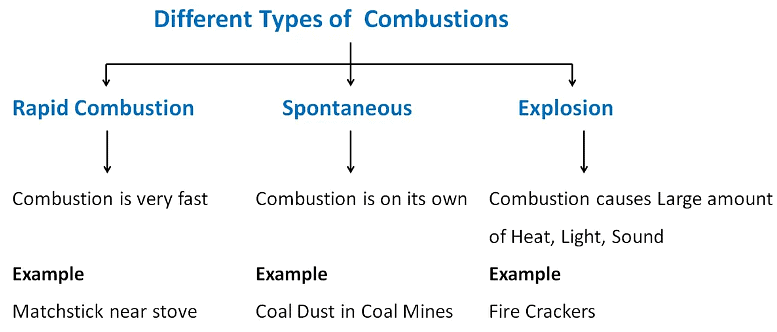
1. Rapid Combustion:
Definition: The combustion reaction in which a large amount of heat and light are produced in a short time is called rapid combustion.
- Rapid energy needs external heat energy for the reaction to occur.
- Combustion produces a large amount of heat and light energy and does so rapidly.
- The combustion will carry on as long as the fuel is available.
- An example is when you light a candle. The heat energy is provided when we light the candle with a matchstick. And it will carry on till the wax burns out. Hence it is a rapid combustion.
2. Spontaneous Combustion
Definition: The combustion reaction which occurs on its own (without the help of any external heat) is called Spontaneous combustion.
- As the name suggests, the combustion occurs spontaneously. This means that it requires no external energy for the combustion to start.
- It happens due to self-heating.
- A substance with low-ignition temperatures gets heated, and this heat is unable to escape.
- In the presence of sufficient oxygen, the temperature rises above the ignition point and combustion will happen.
- The reaction of alkali metals with water is an example.
3. Explosion
Definition: A very fast combustion reaction in which a large amount of heat, light, and sound are produced is called explosive combustion.
- Explosive Combustion happens when the reaction occurs very rapidly.
- A large amount of gas formed in the reaction is liberated. Such a reaction is called an explosion.
- The reaction occurs when something ignites to produce heat, light and sound energy.
- Some classic examples are firecrackers or blowing up of dynamite.
 Explosive Combustion
Explosive Combustion
Flame
When you light a candle a combustion reaction takes place with the wax of the candle which is the fuel and the air which contains oxygen. The flames are produced when fuel vapourises during burning. The flame releases heat and light energy. Hence, this is an exothermic reaction.
A flame can be defined as a region where gaseous elements burn, generating heat and light. All combustible materials which vapourise during combustion, whether liquid or gaseous, emit flames as they burn.
Both the combustible substance and the combustion's supporter must be gases in order for combustion to result in a flame.
Wax, camphor, LPG, magnesium ribbon, candle, kerosene oil, charcoal forms flame on burning.
Structure of flame
Starting from the base of the flame, a flame has four zones.1. Inner Part
- This is the innermost part of the flame.
- It is the part closest to the wick.
- You might assume that this is the hottest part of the flame. However, it is the least hot.
- This is the black part of the flames that contains unburnt particles of the carbon from the wick i.e. unburnt fuel.
2. Middle Part
- This is the biggest part of the flame.
- The colors in this are varying shades of yellow and orange.
- This is the luminous flame because it emits light.
- This part is also not extremely hot. This is because this part gets a limited supply of oxygen.
- Incomplete combustion takes place here which is why it burns orange and is luminous.
3. Outer Part
- This is the hottest part of the flame.
- This part has an unlimited supply of oxygen. So complete combustion takes place here. Hence it is the hottest part of the flame.
- Also, this part of the flames burns with a blue color.
- It is the non-luminous, i.e. does not emit light.
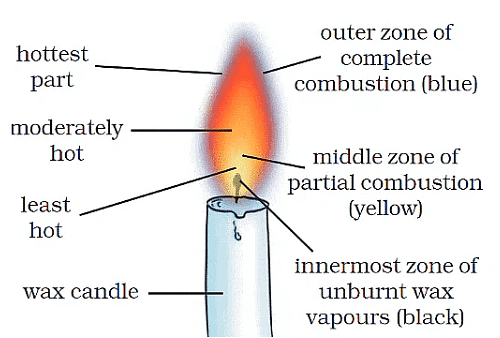 Zones of a Candle Flame
Zones of a Candle Flame
Substances that vaporize while burning produce flames. For instance, kerosene oil and molten wax vaporize through the wick and create flames. In contrast, charcoal doesn't vaporize and thus doesn't produce a flame.
Examples from daily life usage of parts of candle flame:
- When a clean glass plate or slide is introduced into the luminous zone of a steady candle flame and held there for about 10 seconds, a circular blackish ring appears on the plate. This ring shows the deposition of unburnt carbon particles from the luminous part of the flame.
- If you hold a thin copper wire just inside the flame for about 30 seconds, you'll see that the part of the wire outside the flame turns red hot. This indicates that the non-luminous zone of the flame, where the wire is located, has a higher temperature. In fact, this zone is the hottest part of the flame.
- Goldsmiths use the outermost zone of the flame for melting gold and silver because it provides the highest temperature needed for this process.

What is a Fuel?
Any substance that upon combustion produces a usable amount of energy is known as fuel. Heat energy for home and business use primarily comes from sources like wood, charcoal, petrol and kerosene, which are known as fuels.
- A Ideal fuel is one that is not only readily available and inexpensive, but also burns easily and moderately in air, produces a significant amount of heat, and doesn’t leave behind any unwanted substances.
- It’s unlikely that we will find a fuel that can be seen as perfect.
- Rather, we should aim to find a fuel that meets most of the necessary criteria for a specific use.
- Not all fuels have the same cost, with some being more affordable than others.
Examples of Fuel :- Methanol, Gasoline, Diesel, Natural gas, Hydrogen, Biodiesel.
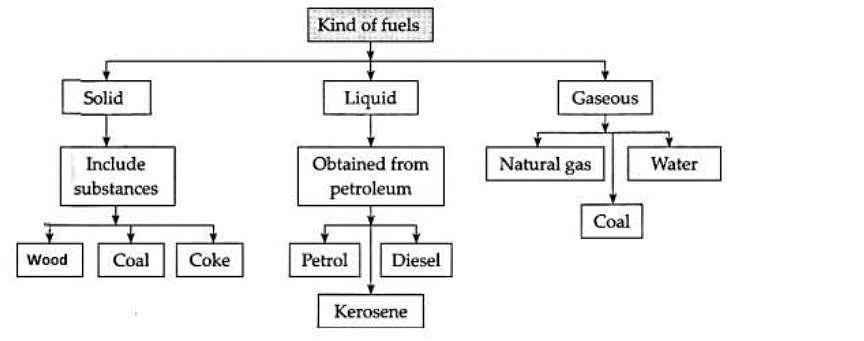
Types of Fuel
1. Solid Fuel
- These are solid materials that combust to produce energy.
- Some examples of Solid fuel are coal, charcoal, soot, wood etc.
- These were most likely the first fuels utilized by mankind.
2. Liquid Fuel
- These are the fuels we burn to produce mechanical energy and kinetic energy.
- Most liquid fuels such as crude oil form due to exposure to intense heat and pressure to fossilized remains of plants and animals
3. Fuel Gas
- Fuel Gas as the name suggests are fuels that are in a gaseous state under normal conditions.
- Some examples are methane, carbon monoxide, propane etc.
- They have an advantage that they can be easily transported to the place of consumption.
Fuel efficiency
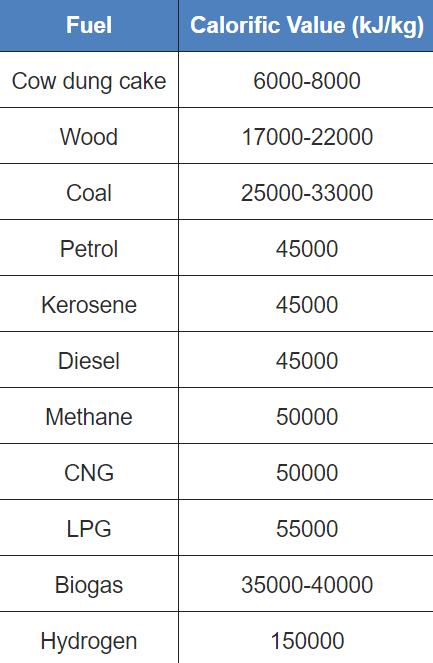
Fuel efficiency is measured as the amount of heat that 1 kg of fuel (any fuel) produces on combustion. This is known as the calorific value of the fuel. The unit of measurement of fuel efficiency is kilojoules per kg, i.e. kJ/kg.
Burning of Fuels Leads to Harmful Products
The rising consumption of fuels has several detrimental effects on the environment:
Pollution from Carbon Fuels: Burning carbon-based fuels such as wood, coal, and petroleum releases unburnt carbon particles. These fine particles are pollutants that can cause respiratory diseases like asthma.
Incomplete combustion of fuels: results in the formation of carbon monoxide, a highly poisonous gas. Burning coal in a confined space is particularly hazardous because the carbon monoxide can accumulate and pose a deadly risk, especially to those who are asleep in the room.
Carbon Dioxide and Global Warming: Most fuels release carbon dioxide during combustion. Higher levels of carbon dioxide in the atmosphere which leads to increased temperatures on Earth is called global warming. This warming causes polar glaciers to melt, resulting in higher sea levels and potential flooding of coastal areas. Some low-lying regions might even become permanently submerged.
Acid Rain: Burning coal and diesel produces sulfur dioxide, a gas that is both suffocating and corrosive. Petrol engines emit nitrogen oxides. These gases dissolve in rainwater, forming acids and creating acid rain, which is harmful to crops, buildings, and soil.
Transition to Cleaner Fuels: To address these environmental issues, there is a shift from diesel and petrol to Compressed Natural Gas (CNG) for vehicles. CNG produces harmful emissions in much smaller quantities, making it a cleaner fuel alternative.
|
92 videos|296 docs|44 tests
|
FAQs on Combustion and Flame Class 8 Notes Science Chapter 4
| 1. What is combustion and how does it occur? |  |
| 2. What are the different types of combustion? |  |
| 3. How can we control fire effectively? |  |
| 4. What is the structure of a flame? |  |
| 5. What is fuel efficiency and why is it important? |  |





















