NCERT Solutions for Class 9 Science Chapter 1 - Matter In Our Surroundings
| Table of contents |

|
| Page No. 3 |

|
| Page No. 6 |

|
| Page No. 9 |

|
| Page No. 10 |

|
Page No. 3
Q1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, the smell of perfume.
Ans: The following substances are matter: Chair, Air, Almonds, Lemon water, and the smell of perfume (The smell is caused by volatile substances which are matter, as they occupy space and have mass).
 Matter around us
Matter around us
Q2. Give reasons for the following observation.
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food, you have to go close.
Ans: When the air is heated, the particles in it gain more kinetic energy and move faster. This is why the smell of hot food travels farther, allowing a person to sense it even from several meters away.
Q3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans: A diver can cut through water in a swimming pool. This shows that the particles of water have intermolecular space and have less force of attraction. Thus, water molecules flow easily, giving way to a diver.
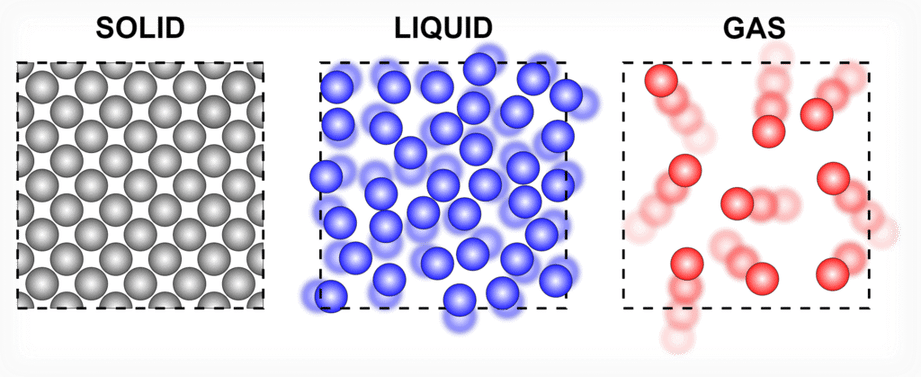 Showing less intermolecular force between liquid molecules.
Showing less intermolecular force between liquid molecules.
Q4. What are the characteristics of the particles of matter?
Ans: The characteristics of particles of matter are:
(i) Presence of intermolecular spaces between particles.
(ii) Particles are in constant motion.
(iii) They attract each other.
Page No. 6
Q1. The mass per unit volume of a substance is called density.
(density=mass/volume). Arrange the following in the order of increasing density – air, exhaust from the chimneys, honey, water, chalk, cotton and iron.
Ans: The following substances are arranged in increasing density: Air < Exhaust from chimney < Cotton < Water < Honey < Chalk < Iron.
Q2. (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Ans: (a) The differences in the characteristics of the three states of matter solid, liquid and gas are:-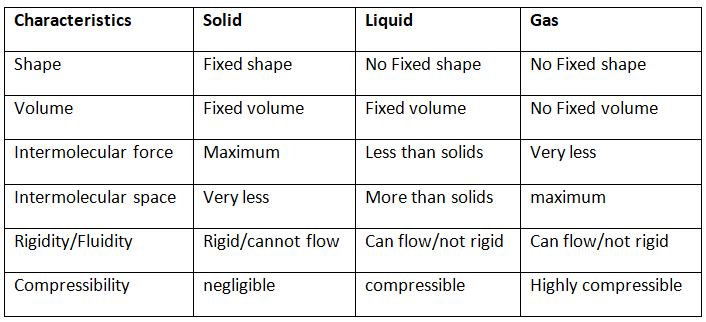
(b)
(i) Rigidity: It is the property of matter that continues to remain in its shape when treated with an external force.
(ii) Compressibility: Particles have the ability to reduce their intermolecular space when an external force is applied, which increases their density. This characteristic is called compressibility.
(iii) Fluidity: It is the ability of a substance to flow or move about freely.
(iv) Filling the gas container: The particles in a container take their shape as they randomly vibrate in all possible directions.
(v) Shape: It is the definite structure of an object within an external boundary.
(vi) Kinetic energy: Motion allows particles to possess energy, which is referred to as kinetic energy. The increasing order of kinetic energy possessed by various states of matter is Solids < Liquids < Gases.
Mathematically, it can be expressed as K.E = 1/2 mv2, where ‘m’ is the mass and ‘v’ is the velocity of the particle.
(vii) Density: It is the mass of a unit volume of a substance. It is expressed as d = M/V, where ‘d’ is the density, ‘M’ is the mass and ‘V’ is the volume of the substance
Q3. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in the air, but to do the same through a solid block of wood, we need a karate expert.
Ans:
(a) There is a low force of attraction between gas particles. The particles in the filled vessel are free to move about.
(b) Gaseous particles have the weakest attraction force and move randomly in all directions. When a gas particle hits the walls of its container, it applies a force, creating pressure on the walls.
(c) The hardwood table has a definite shape and volume. The wood particles are tightly packed and do not change to fit the shape of a container. This gives the table its solid properties.
(d) The air particles are spread far apart with a lot of space between them, which is why we can move our hands freely through the air. However, in a solid block, particles are tightly packed with negligible free space, leaving little or no space between them. That's why breaking a solid block would require the strength of a karate expert.
Q4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why?
Ans:
- The mass per unit volume of a substance is called density (density = mass/volume).
- As the volume of a substance increases, its density decreases. In general, solids have higher density than liquids. Water is also a liquid, so it should also have less density than that of a solid, which is ice.
- Though ice is solid, it has a cage-like structure; hence, there are a large number of empty spaces between its particles. These spaces are larger than the spaces between the particles of water. Thus, for a given mass of water, the volume of ice is greater than that of water.
- Hence, the density of ice is less than that of water. A substance with a lower density than water can float on water. Therefore, ice floats on water.
Page No. 9
Q1. Convert the following temperature to Celsius scale:
(a) 300 K
(b) 573 K
Ans: To convert a temperature from the Kelvin scale to the Celsius scale, you simply subtract 273 from the given Kelvin temperature.
(a) (300 - 273)°C = 27°C
(b) (573 - 273)°C = 300°C
Q2. What is the physical state of water at:
(a) 250°C
(b) 100°C
Ans:
(a) At 250°C, the physical state of water is gas as the temperature is beyond its boiling point.
(b) At 100°C, it is in the transition state (both liquid and gaseous states) as the water is at its boiling point. Hence, it would be present in both liquid and gaseous state.
Q3. For any substance, why does the temperature remain constant during the change of state?
Ans: It is due to the latent heat as the heat supplied to increase the temperature of the substance is used up to transform the state of matter of the substance; hence, the temperature stays constant. Latent heat is the heat energy needed to change a substance from one form to another without changing its temperature. For example, when ice melts into water, it absorbs heat (latent heat of fusion) without increasing in temperature. Similarly, when water evaporates into steam, it also absorbs heat (latent heat of vaporization) without a temperature change.
Q4. Suggest a method to liquefy atmospheric gases.
Ans: To transform a gas into a liquid, it is necessary to bring its constituent particles or molecules closer. This can be achieved with atmospheric gases by either increasing the pressure or lowering the temperature.
Page No. 10
Q1. Why does a desert cooler cool better on a hot dry day?
Ans: A desert cooler works better on hot, dry days because the high temperature and low humidity increase the rate of evaporation. This greater evaporation results in more effective cooling.
Q2. How does the water kept in an earthen pot (matka) become cool during summer?
Ans: An earthen pot has tiny pores that allow water to seep through and evaporate from its surface. This evaporation requires energy, which is drawn from the water inside the pot, making the water cooler.
Q3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Ans: Acetone, petrol, and perfume are highly volatile substances. When applied to our palm, they evaporate quickly, absorbing heat from the skin and making our palm feel cold.
Q4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Ans: A saucer has a larger surface area than a cup, which allows for faster evaporation. This rapid evaporation cools the tea or milk more quickly, enabling us to sip it faster.
Q5. What type of clothes should we wear in summer?
Ans: In summer, it is advisable to wear light-colored cotton clothes. Light colors reflect heat, and cotton is breathable, allowing sweat to evaporate and providing a cooling effect on the skin.
Exercises
Q1. Convert the following temperature to Celsius scale.
(a) 293K
(b) 470K
Ans: To convert a temperature from the Kelvin scale to the Celsius scale, you simply subtract 273 from the given Kelvin temperature because 0°C=273K.
(a) 293K= (293 – 273)°C = 20°C
(b) 470K= (470 – 273)°C = 197°C
Q2. Convert the following temperatures to the kelvin scale.
(a) 25°C
(b) 373°C
Ans:
0°C = 273K
(a) 25°C = (25+273)K = 298K
(b) 373°C = (373+273)K = 646K
Q3. Give reason for the following observations:
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Ans:
(a) At room temperature, naphthalene balls undergo sublimation, which means they change directly from a solid to a gaseous state without undergoing the intermediate state, i.e., the liquid state.
 Naphthalene Balls
Naphthalene Balls
(b) It is because perfumes contain a volatile organic solvent that can easily diffuse through air and, hence, carry the fragrance to people sitting several metres away.
Q4. Arrange the following substances in increasing order of forces of attraction between the particles— water, sugar, oxygen.
Ans: Oxygen (gas) < Water (liquid) < Sugar (solid)
Q5. What is the physical state of water at:
(a) 25°C, (b) 0°C, (c) 100°C?
Ans: The physical state of water at different temperatures is as follows:
(a) At 25°C, water is in a liquid state (typical room temperature).
(b) At 0°C, water is at its freezing point, so both solid (ice) and liquid (water) phases can be observed.
(c) At 100°C, water is at its boiling point, resulting in the presence of both liquid water and gaseous water (water vapor).
Q6. Give two reasons to justify.
(a) Water at room temperature is a liquid.
(b) An iron almirah is a solid at room temperature.
Ans:
(a) Water remains in a liquid state at room temperature for two main reasons:
- Its melting or freezing point is lower than room temperature, while its boiling point is higher (100°C).
- Water has no fixed shape and flows to take the shape of its container, which indicates that it occupies a fixed volume but does not have a defined shape.
(b) An iron almirah is a solid at room temperature for the following reasons:
- Both the melting and boiling points of iron are above room temperature, meaning it remains solid under these conditions.
- An iron almirah is rigid and maintains a definite shape, and metals typically have a high density, further confirming that it is solid at room temperature.
Q7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Ans: Ice at 273 K will absorb heat energy, equivalent to the latent heat of fusion from the surroundings. The water at the same temperature does not absorb this heat energy. Therefore, the cooling effect of ice is more than water at 273 K.
Q8. What produces more severe burns, boiling water or steam?
Ans: Steam has more energy than boiling water. It possesses the additional latent heat of vaporisation. Therefore, burns produced by steam are more severe than those produced by boiling water.
Q9. Name A, B, C, D, E and F in the following diagram showing change in its state.
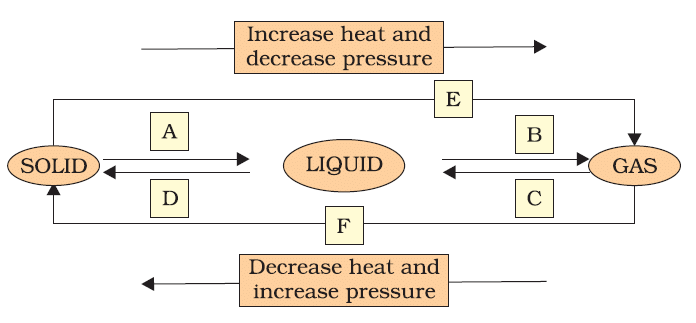 Ans: Interconversion of three states of matter: Using temperature or pressure, any state of matter can be turned into another.
Ans: Interconversion of three states of matter: Using temperature or pressure, any state of matter can be turned into another.
(A) Solid to Liquid → Melting (or) fusion (or) liquefaction
(B) Liquid to Gas → Evaporation (or) vaporization
(C) Gas to liquid → Condensation
(D) Liquid to Solid → Solidification
(E) Solid to Gas → Sublimation
(F) Gas to Solid → Deposition
|
84 videos|543 docs|60 tests
|
FAQs on NCERT Solutions for Class 9 Science Chapter 1 - Matter In Our Surroundings
| 1. What is matter and how is it classified? |  |
| 2. What are the different states of matter? |  |
| 3. How do temperature and pressure affect the state of matter? |  |
| 4. What is the difference between evaporation and boiling? |  |
| 5. How can we separate mixtures? |  |
















