Class 9 Science Chapter 1 Question Answers - Matter in Our Surroundings (Not in 2021-22 Syllabus)
Ques1. We can smell the perfume sitting several meters away. Why?
Ans. The ability to smell perfume from several metres away is due to the presence of volatile solvents in the fragrance. These solvents:
- Release pleasant-smelling vapours into the air.
- Diffuse quickly, allowing the scent to travel.
- Reach people even at a distance, making the aroma detectable.
Ques2. Why can you smell the perfume of incense sticks?
Ans. The particles of the perfume from incense sticks are not still; they are always moving. This movement allows them to drift through the air, making it possible for us to smell the fragrance. Incense Stick
Incense Stick
Ques3. Why can not you smell its perfume at a short distance when the incense stick is not lighted ?
Ans. The particles of the perfume do not have enough energy to move through the air when the incense stick is unlit. As a result:
- The scent cannot be detected from a distance.
- Only when the stick is lit do the particles gain energy.
- This allows the fragrance to spread and be smelled from farther away.
Ques4. Why is the smell of the perfume of incense stick fill the whole room in a few minutes, when lighted?
Ans. When the incense stick is lighted, the heat energy makes the particles of the perfume to move rapidly. Thus, they easily drift through the air in the room and hence we can smell it anywhere in the room.
Ques5. A rubber band is a solid, but it can change its shape. Why ?
Ans. A rubber band is considered a solid because it can return to its original shape after being stretched. Here are some key points:
- When a stretching force is applied, the rubber band changes its shape.
- Once the force is removed, it regains its original shape.
- If too much force is applied, the rubber band can break.
Ques6. When salt or sugar are poured into different kinds of vessels, why do they take the shape of vessels ?
Ans. Salt or sugar takes the shape of a containing vessel, but does not change its individual shape. For example, sugar crystals are cubical, and they remain cubical in any vessel.
Ques7. Sponge is a solid, yet we are able to compress it. Why?
Ans. Sponge has very small holes throughout its structure. These holes are filled with air. When it is compressed, the air in the holes is squeezed out. Thus, we are able to compress it. Sponge
Sponge
Ques8. Arrange the following substances in the increasing order of forces of attraction between the particles water, sugar and oxygen.
Ans. In terms of the forces of attraction between particles, the substances can be arranged as follows:
- Oxygen - has the weakest attraction between particles.
- Water - has a moderate attraction.
- Sugar - has the strongest attraction among the three.
Thus, the increasing order is: Oxygen > Water > Sugar.
Ques 9. What is the physical state of water at:
(a) 25°C
(b) 0°C
(c) 100°C
Ans.
(a) At 25°C, water is in a liquid state.
(b) At 0°C, water is in solid state, provided heat is removed from it.
(c) At 100°C, water is in a gaseous state, provided heat is supplied to it.
Ques10. Give two reasons to justify.
(a) Water at room temperature is a liquid.
(b) An iron almirah is solid at room temperature.
Ans.
(a) Water at room temperature is a liquid because:
- The temperature of 25°C is above the freezing point of water, which is 0°C. At this temperature, water molecules have enough energy to move freely, allowing it to exist as a liquid.
- In its liquid state, water maintains a definite volume but takes the shape of its container, which is characteristic of liquids.
(b) An iron almirah is a solid at room temperature because:
- It has a fixed shape and volume, which are typical properties of solids.
- The particles in an iron almirah are closely packed together, providing it with structural integrity and rigidity.
 Iron almirah
Iron almirah
- Thus, the molecules are held very, very tightly, with the result, the iron almirah has a definite shape and definite shape and definite volume, and hence, is a solid.
Ques11. State your observation immediately after adding the blue ink drop.
Ans. After adding the drop of blue ink, the following observations can be made:
- The ink begins to trickle down the sides of the beaker.
- As it moves, the blue colour starts to diffuse into the water.
- This creates wavy blue streaks throughout the liquid.
 Diffusion of ink in water
Diffusion of ink in water
Ques12. State your observation immediately after adding the honey drop.
Ans. After adding the drop of blue ink, the following observations can be made:
- The ink begins to trickle along the sides of the beaker.
- As it moves, the blue colour starts to diffuse into the water.
- This creates wavy blue streaks throughout the liquid.
Ques13. How much time does it take for the colour of ink to spread evenly?
Ans. The ink spreads evenly in water in approximately two hours.
Ques14. How does the diffusion of honey vary with the diffusion of ink and why ?
Ans. The diffusion of honey is very slow as compared to the diffusion of ink. It is because the honey is a dense liquid. Its particles have strong intermolecular forces as compared to water. Thus, it diffuses slowly in water.
Example:
- Add few crystals of sugar to water they intermix (dissolve) with water spontaneously.
- When we add a few drops of ink to water, the colour of the ink gets dispersed evenly in the entire liquid.
The gases also diffuse into liquids:
- Aqueous solution of ammonia contains ammonia diffused in water.
- The gases from the atmosphere diffuse and dissolve in water especially O2, CO2 are essential for the survival of aquatic animals and plants.
- The fish and other aquatic animals can utilize the dissolved oxygen for producing energy form food.
 Aquatic Life
Aquatic Life
Ques15. What happens around each crystal of solid on introducing it to water ?
Ans. When a crystal is introduced to water, a dense and deep violet colour forms around it. The size of this violet area is larger in hot water compared to cold water.
Ques16. What happens as the time passes, and why?
Ans. The dense violet colour begins to diffuse into cold water, forming coloured streaks. Over time, the solution turns pink, with a darker hue near the base of the beaker. In contrast, when added to hot water, the violet colour diffuses rapidly, resulting in a more homogeneous pink solution compared to that in cold water.
Ques17. Does the rate of diffusion change with temperature? If so, why?
Ans. The rate of diffusion increases with temperature due to the following reasons:
- Higher temperature means that particles have more kinetic energy.
- Increased kinetic energy causes particles to move faster.
- As particles move faster, they can spread out more quickly into the surrounding space.
- This results in a quicker rate of diffusion, such as when solid potassium permanganate is placed in hot water.
 Potassium permanganate crystals in water
Potassium permanganate crystals in water
Ques 18. What do you observe on the sides of the glass beaker?
Ans. Tiny bubbles of gas cling to the sides of the beaker.
Ques 19. Give an explanation to your above observation.
Ans.
- The tiny bubbles are of air (especially carbon dioxide and oxygen) which got dissolved in water naturally.
- These gases are expelled when water is warmed.
- The gases like oxygen and carbon dioxide diffuse and hence dissolve in water. The dissolved oxygen in water is essential for the respiration of water animals.
- The dissolved carbon dioxide helps the water plant to synthesize their food by the process of photosynthesis.
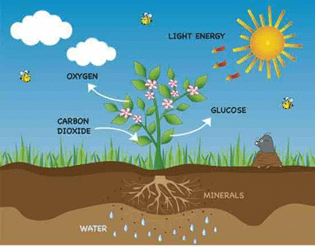 Photosynthesis
Photosynthesis
Ques 20. What do you observe when force is applied and then removed on the plunger of the syringe containing water ? Give a reason for your answer.
Ans. The plunger does not move inward on the application of force. When the force is removed, the plunger does not move backward.
Reason: The liquids have small intermolecular spaces. Thus, they cannot be compressed.
Ques 21. What do you observe when force is applied and then removed on the plunger of the syringe containing air ? Give a reason for your answer.
Ans. The plunger moves downward on the application of force to a considerable length. When the force is removed, the plunger moves backward and takes its original position.
Reason: The gases have large intermolecular spaces. Thus, they easily get compressed on the application of force. The compressed gases are under high pressure. When the force is removed, this high pressure forces the plunger back to its original position.
Ques 22. Give reasons:
(a) A gas fills the vessel in which it is kept completely.
Ans. The molecules of a gas have large intermolecular spaces and kinetic energy, but extremely small intermolecular forces. Thus, the molecules of the gas spread in the entire space of the containing vessel on account of high kinetic energy and practically to intermolecular forces, hence filling entire space of the vessel.
(b) A gas exerts pressure on the walls of the container.
Ans. The molecules of a gas have very large kinetic energy. When these molecules strike against the walls of a containing vessel, they exert a certain average force per unit area. As the force per unit area is known as pressure, therefore, the gases exert pressure on the sides of the containing vessel.
(c) A wooden table should be called a solid.
Ans. Solids are rigid, incompressible, and have definite shape and volume. Since the table has all the above mentioned properties, therefore, it it solid.
 Particles in solids
Particles in solids
(d) We can easily move our hand in the air, but to do the same through a solid block of wood, we need a karate expert.
Ans. The intermolecular forces between the molecules of a gas are almost negligible and intermolecular spaces are very large. Thus, we can easily move our hand in air, without any appreciable force.
The intermolecular forces between the molecules of a solid are very large and intermolecular spaces are very small. Thus, a lot of force is required to separate the molecules of a solid. It is for the same reasons that we need karate expert to break a block of wood.
Ques 23. The mass per unit volume of a substance is called density. (Density = Mass / Volume). Arrange the following in the order of increasing density: air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Ans. Exhaust from chimneys, air, cotton, water, honey and iron.
Example:
(i) CNG (compressed Natural gas) is used as fuel in internal combustion engines.
(ii) Oxygen in compressed form is supplied to hospitals for serious patients in cylinders.
(iii) LPG (Liquefied petroleum gas) which is used in home for cooking.
Ques 27. The diver is able to cut through water in a swimming pool.
Ans. The diver can cut through water in a swimming pool due to the following reasons:
- The particles in water are not tightly packed; there are spaces between them.
- The attractive forces between water molecules are relatively weak.
- When the diver applies force, they displace the water, allowing them to move through it.
Ques 28. Why does ice float on water?
Ans. Ice floats on water because of its unique structure. Here are the key points:
- Typically, solids are denser than liquids.
- However, ice has a specific structure that creates larger spaces between its particles.
- This structure results in a lower density compared to liquid water.
- As a result, ice is able to float on water.
 Ice floats on water
Ice floats on water
Ques 29. Convert the following temperatures to the celsius scale.
(a) 300 K
(b) 573 K
Ans.
(a) (300 - 273) = 27°C. Temperature in °C = Temperature in K -273
(b) (573 - 273) = 300°C. Temperature in °C = Temperature in K -273
Ques 30. Convert the following temperature to the Kelvin scale.
(a) 25°C
(b) 373°C
Ans.
(a) 25 + 273 = 298 K
(b) 373 + 273 = 646 K
Ques 31. Ice is at 273 K more effective in cooling, than water at the same temperature, why?
Ans. Ice at 273 K cools more effectively than water because:
- Ice absorbs heat energy, known as latent heat, from its surroundings to melt into water.
- This absorption of heat occurs without a rise in temperature, enhancing its cooling effect.
- In contrast, water at the same temperature does not absorb this extra heat, making it less effective for cooling.
Ques 32. What produces more severe burns, boiling water or steam?
Ans. Steam causes more severe burns than boiling water. This is due to the following reasons:
- 1 gram of steam at 373 K (100°C) contains 2260 J of heat energy.
- This energy is in the form of latent heat of vaporisation, which is significantly higher than that of boiling water at the same temperature.
- As a result, steam can transfer more energy to the skin, leading to more severe burns.
Ques 33. What is the physical state of water at:
(a) 25°C
(b) 0°C
(c) 100°C
Ans.
(a) 25°C - Water is in a liquid state.
(b) 0°C - Water is in solid state.
(c) 100°C - Water is in a gaseous state.
Ques 34. Naphthalene balls disappear with time without leaving any solid why
Ans. Naphthalene balls disappear over time without leaving any solid residue because they are volatile and sublime.
- Volatile: Naphthalene easily transitions from solid to gas at room temperature.
- Sublimation: This process allows the solid to change directly into vapour without becoming a liquid.
 Naphthalene Balls
Naphthalene Balls
Therefore, it changes into vapors completely, which disappear into the air, and no solid is left.
|
84 videos|478 docs|60 tests
|
FAQs on Class 9 Science Chapter 1 Question Answers - Matter in Our Surroundings (Not in 2021-22 Syllabus)
| 1. What are the different states of matter? |  |
| 2. How does matter change from one state to another? |  |
| 3. What is the difference between evaporation and boiling? |  |
| 4. How does temperature affect the state of matter? |  |
| 5. Can matter exist in more than three states? |  |

















