S-block Elements :
The elements in which the last electron enters the outermost s-orbital are called s-block elements. The group 1 and 2 of periodic table belong to the s-block.
Group-I Elements (Alkali Metals) :
(1) The Elements : are Li, Na, K, Rb, Cs Fr (Radioactive: t1/2 of Fr233 = 21 minutes)
group-I elements are called alkali metals because they form hydroxides on reaction with water, which are alkaline in nature.
(2) Outer Electronic configuration : ns1 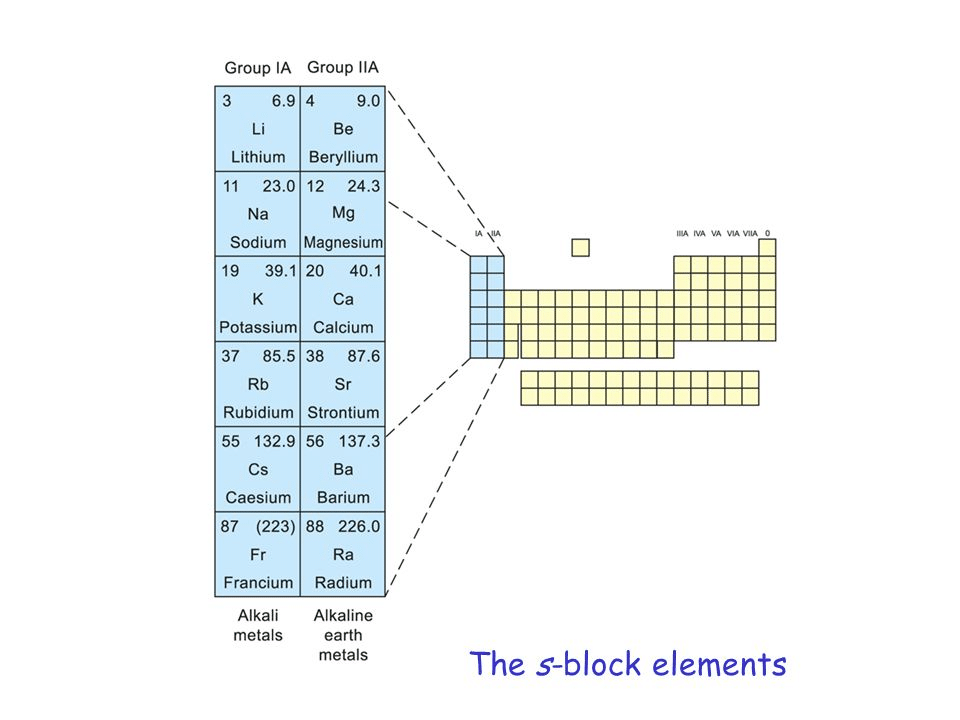
(3) Atomic and Ionic radii :
Li < Na < K < Rb < Cs.
Increase down the group, because value of n(principal quantum number) increases.
(4) Density :
Li < K < Na < Rb < Cs.
(5) Ionization Energy :
Li > Na > K > Rb > Cs.
As size increases, I. E. decreases down the group (so Cs have lowest I. P. )
(6) Hardness and melting points/boiling points :
These metals are very soft and can be cut with a knife. Litium is harder than any other alkali metal.
The hardness depends upon cohesive energy
M. P. Li > Na > K > Rb > Cs
B. P. Li > Na > K > Cs > Rb
(7) Electropositive character or metallic character :
Alkali metals are strongly electropositive and metallic. Down the group electropositive nature increase so metallic nature also increases.
i.e., M → M e-
Metallic Nature : Electropositive character a 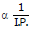
Li < Na < K < Rb < Cs.
(8) Oxidation state :
Show 1 oxidation state because by loosing one electron they get stable noble gas configuration.
(9) Photoelectric effect :
The phenomenon of emission of electrons when electromagnetic rays strikes against them is called photoelectric effect ; Alkali metal have low I. P. so show photoelectric effect.
*Cs and K are used in photoelectric cells.
Chemical Properties :
(1) Reactions with air
The alkali metals tarnish in dry air due to the formation of their oxides on their surface, which in turn react with water to form hydroxides
4M + O2 → 2M2O
M2O + H2O → 2MO
They react vigorously in oxygen forming following oxides.
4 Li + O2 → 2 Li2O (Monoxide)
2 Na + O2 → Na2O2(Peroxide)
M + O2 → MO2(Superoxide) where M = K, Rb, Cs
(2) Solution in liquid NH3
Alkali metals dissolve in liquid ammonia (high conc. 5 M) and give blue solution which is conducting, reducing and paramagnetic in nature.
Reason
On dissolving Metal in NH3
M(s) M e-
M + x(NH3) → [M(NH3)x] Ammmoniated cation
e- + y(NH3) → [e(NH3)y]- Ammmoniated electron
The blue colour is due to → Ammoniated electron
The paramagnetic nature is due to → Ammoniated electron
The conducting nature is due to → Ammoniated M Ammoniated electron
* On standing the colour fades due to formation of amide
M + e- + NH3 → MNH2(amide) + H2(g)
In the absence of impurities like. Fe, Pt, Zn etc, the solutions are stable.
* In concentrated solution, the blue colour changes to bronze colour and diamagnetic due to the formation of metal clusters and ammoniated electrons also associate to form electron pairs
2e-(NH3)y → [e_(NH3)4]2
(3) Reducing Nature
Reducing agent is electron donor.
Alkali metals are strong reducing agents with lithium being the strongest and sodium the least prowerful reducing agent. Na < K < Rb < Cs < Li
Note : Lithium is expected to be least reducing agent due to it's very high IE. However it is strongest. (due to high hydration energy).
(4) Reaction with H2O
The reaction with water to form the hdyroxides having the formula MOH
2M + 2H2O → 2MOH H2
(Highly reactive)
(5) Reaction with H2
They react with H2 forming metal hydride with formula MH which are of ionic nature. Stability of hydride decreases down the group.
(6) Reaction with N2
Only Lithium reacts with N2 to form ionic lithium nitride Li3N.
3Li + N2 → Li3N
(7) Reaction with halogens X2
The alkali metals react vagariously with halogens to form ionic halides M X-
2M + X2 → 2MX
(8) Sulphides
All metals react with S forming sulphides such as Na2S and Na2Sn(n = 2,3,4,5 or 6). The polysulphide ions are made from zig-zag chains of sulphur atoms.
(9) Crown Ethers and Cryptands
Dibenzo-18-Grown-6 Cryptand-222
[Na(Cryptand 222)] Na- [Contains Na_(sodide ion)]
[(s (Cryptand-222)] [(Cyrptand-222)e-] [electride]
Group II Elements (Alkaline earth Metals) :
(1) Elements : The Elements are Be, Mg, Ca, Sr, Ba, Ra,
(2) Outermost Electronic configuration : ns2 ,
(3) Atomic and ionic sizes :
* The atomic and ionic radii of the alkali earth metal are smaller than corresponding alkali metals
Reason : higher nuclear charge (Zeff)
* On moving down the group size increase, as value of n increases.
Be < Mg < Ca < Sr < Ba
(4) Ionization Enthalpy :
Be > Mg > Ca > Sr > Ba
Down the group IE decreases due to increase in size
Q. IE1 of AM < IE1 of AEM
IE2 of AM > IE2 of AEM
[Where AM = Alkali metal, AEM = Alkaline earth metal]
Reason : IE1 to AEN is large due to increased nuclear charge in AEM as compared to AM but IE2 of AM is large because second electron in AM is to be removed from action which has already acquired noble gas configuration.
(5) Melting and Boiling points :
They have low m.p. and b.p. but are higher than corresponding value of group I.
Reason : They have two valency electrons which may participate in metallic bonding compared with only one electron in AM. Consequently group II elements are harder and have higher cohesive energy and have much higher m.p./b.p. than A. M.
M.P. Be > Ca > Sr > Ba > Mg
B.P. Be > Mg > Ca > Ba > Sr
(6) Electropositive and Metallic character :
Due to low IE they are strong electropositive but not as strong as AM because of comparatively high IE.
The electropositive character increase down the group.
Be < Mg < Ca < Sr < Ba
(7) Oxidation state : Show 2 oxidation state.
Chemical Properties :
(1) Reactivity towards Air or Oxygen
Be and Mg are kinetically inert towards oxygen because of formation of a film of oxide on their surface.
However powdered Be burn brilliantly.
2 Be + O2(air) 2BeO
3 Be + N2(air) Be3N2
Only Mg give the follwoing behaviour.
Mg + Air(N2 + O2) MgO + Mg3N2
(Similar property with Li due to diagonal relation.)
* BeO, MgO are used as refractory, because these have high m.p.
* Other metals (Ba or Sr form peroxide)
M + O2 MO2
(2) Reaction with H2O
AEM have lesser tendency to react with water as compared to AM. They form hydroxides and liberate H2 on reaction with H2O.
M + 2H2O M(OH)2 + H2
* Be is inert towards water.
* Magnesium react as
Mg + 2H2O → Mg(OH)2 + H2
or Mg + H2O → MgO + H2O
MgO forms protective layer, that is why it does not react readily unless layer is removed amalgamating with Hg.
Other metals react quite readily (Ca, Sr, Ba).
Note : Be(OH)2 is amphoteric but other hydroxides are basic in nature.
(3) Reaction with Acids & Bases
AEM react with acids & liberate H2
Mg + 2HCl → MgCl2 + H2
Be is amphoteric as it also react with NaOH, other metals do not react as they are purely basic.
Be + 2NaOH → Be(OH)2 [Be(OH)4]2_
(4) Tendency to form Complexes
AEM have tendency to form some stable complexes. Among these Be and Mg have maximum tendency due to their small size and high charge density.
BeF2 + 2F- →` [BeF4]-2
*Chlorophyll contains Mg2 [Photosynthetic pigment in plants]
*Ca2 and Mg2 form complex with EDTA.
(5) Reactivity with NH3
Like AM, the AEM (only Ca, Sr, Ba) dissolve in by NH3 to give deep blue -black solutions having ammoniated cations, and ammoniated electrons.
(6) Reaction with Carbon
AEM when heated with carbon from carbides
*Be form Be2C
*Mg, Ca, Sr, Ba form carbides of the formula MC2.
Group - I & II :
Oxides :
Sodium Oxide (Na2O) :
Preparation :
(i) It is obatined by burning sodium at 180ºC in a limited supply of air or oxygen and distilling off the excess of sodium in vacuum.
2Na + O2
Na2O
(ii) By heating sodium peroxide, nitrate or nitrite with sodium.
Na2O2 + 2Na → 2Na2O
2NaNO3 + 10Na → 6Na2O N2
2NaNO2 + 6Na → 4Na2O N2
Properties :
(i) It is white amorphous mass.
(ii) It decomposes at 400ºC into sodium peroxide and sodium
2Na2O Na2O2 + 2Na
(iii) It dissolve violently in water, yielding caustic soda.
Na2O + H2O → 2NaOH
Sodium Peroxides (Na2O2) :
Preparation : It is formed by heating the metal in excess of air or oxygen at 300º, which is free from moisture and CO2.
2Na + O2 → Na2O2
Properties :
(i) It is a pale yellow solid, becoming white in air from the formation of a film of NaOH and Na2CO3.
(ii) In cold water (~ 0ºC) produces H2O but at room temperature produces O2. In ice-cold mineral acids also produces H2O2.
Na2O2 + 2H2O 2 NaOH + H2O2
2Na2O2 + 2H2O 4 NaOH + O2
Na2O2 + H2SO4 Na2SO4 + H2O2
(iii) It reacts with CO2, giving sodium carbonate and oxygen and hence its use for purifying air in a confined space e.g. submarine, ill-ventilated room,
2Na2O2 + 2CO2 → 2Na2CO3 + O2
(iv) It is an oxidising agent and oxidises charcoal, CO, NH3, SO2
3Na2O2 + 2C → 2Na2CO3 + 2Na [Deposition of metallic Na]
CO + Na2O2 → Na2CO3
SO2 + Na2O2 → Na2SO4
2NH3 + 3Na2O2 → 6NaOH + N2
(v) It containes peroxide ion [-O-O-]-2
Uses :
(i) For preparing H2O2, O2,
(ii) Oxygenating the air in submarines,
(iii) Oxidising agent in the laboratory.
Oxides of Potassium :
K2O, K2O2 , K2O3, KO2, KO3
Colours : White White Red Bright Yellow Orange Solid
Preparation :
(i) 2KNO3 + 10 K 6K2O + N2
** K2O K2O
(White) (Yellow)
** K2O + H2O 2KOH
(ii) 2K + O2 K2O2 [Props : Similar with Na2O2]
(iii) Passage of O2 through a blue solution of K in liquid NH3 yields oxides K2O2(white), K2O3 (red) and KO2 (deep yellow) i.e.
K in liq. NH3 K2O2
K2O3
KO2
white red yellow
** KO2 reacts with H2O and produces H2O2 and O2 both
2KO2 + 2H2O 2KOH + H2O O2
KO3 : KOH O3 (ozonised oxygen) KO3
(Dry powdered) (orange solid)
Magesium Oxide (MgO) :
It is also called magnesia and obtained by heating natural magnesite.
MgCO3 MgO + CO2
Properties :
(i) It is white powder
(ii) It's m.p. is 2850ºC. Hence used in manufacture of refractory bricks for furances.
(iii) It is very slightly soluble in water imparting alkaline reaction.
Calcium Oxide (CaO) :
It is commonly called as quick lime or lime and made by decomposing lime stone at high temperature about 1000ºC.
CaCO3 CaO + CO2 42000 cal
Properties :
(i) It is white amorphous powder of m.p. 2570ºC.
(ii) It emits intense light (lime light), when heated in oxygen - hydrogen flame.
(iii) It is an basic oxide and combines with some acidic oxide e.g.
CaO + SiO2 CaSiO3
CaO + CO2 CaCO3
(iv) It combines with water to produce slaked lime.
CaO + H2O Ca(OH)2
Magnesium Peroxide (MgO2) and Calcium Peroxide (CaO2) :
These are obtained by passing H2O2 in a suspension of Mg(OH)2 and Ca(OH)2.
Uses : MgO2 is used as an antiseptic in tooth paste and as a bleaching agent.
Hydroxides :
Sodium Hydroxides :
Preparation :
(i) Electrolysis of Brine :
NaCl Na Cl-
At anode : 2Cl- Cl2 2e
At cathode : H + e- H
(ii) Caustication of Na2CO3 (Gossage's method) :
Na2CO3 + Ca(OH)2 ⇔ 2NaOH + CaCO3 (precipitate)
(suspension)
Since the Ksp(CaCO3) < Ksp(Ca(OH)2), the reaction shifts towards right.
Properties :
(i) It is white crystalline, deliquescent, highly corrosive solid.
(ii) It is stable towards heat.
(iii) It's aqueous solution alkaline in nature and soapy in touch.
(iv) NH4Cl + NaOH NaCl + NH3 + H2O
FeCl3 + 3NaOH Fe(OH)3
+ 3NaCl
Brown ppt
ZnCl2 + 2NaOH Zn(OH)2
+ 2NaCl
Zn(OH)2+ 2NaOH
Na2ZnO2 + 2H2O [Same with AlCl3, SnCl2, PbCl2]
soluble.
(v) Acidic and amphoteric oxides gets dissolved easily e.g.
CO2 + 2NaOH Na2CO3 + H2O
Al2O3 + 2NaOH 2NaAlO2 + H2O
(vi) Aluminium and Zn metal gives H2 from NaOH
2Al + 2NaOH + 2H2O 3H2 + 2NaAlO2
(vii) Several non metals such as P, S, Cl etc. yield a hydride instead of hydrogen e.g.
4P + 3NaOH + 3H2O PH3 + 3NaH2PO2 (Disproportionation reaction)
Potassium Hydroxide :
Preparation : Electrolysis of KCl aqueous solution
Properties : Same as NaOH
**(a) It is stronger base comapred to NaOH.
(b) Solubility in water is more compared to NaOH.
(c) In alcohol, NaOH is sparingly soluble but KOH is highly soluble.
(d) As a reagent KOH is less frequently used but in absorption of CO2, KOH is preferably used compared to NaOH. Because KHCO3 formed is soluble whereas NaHCO3 is insoluble and may therefore choke the tubes of apparatus used.
Magnesium Hydroxide : It occurs in nature as the mineral brucite.
Preparation : It can be prepared by adding caustic soda solution to a solution of Mg-sulphate or chloride soltion.
Mg+2 + 2NaOH Na2SO4 + Mg(OH)2
Properties :
(i) It can be dried at temperature upto 100ºC only otherwise it breaks into its oxide at higher temperature. Mg(OH)2 MgO + H2O
(ii) It is slightly soluble in water imparting alkalinity.
(iii) It dissolves in NH4Cl solution
Mg(OH)2 + 2NH4Cl MgCl2 + 2NH4OH
** Thus, Mg(OH)2 is not therefore precipitated from a solution of Mg 2 ions by NH4OH in presence of excess of NH4Cl.
Calcium Hydroxide :
Preparation : By spraying water on quicklime
CaO + H2O Ca(OH)2
Properties :
(i) It is sparingly soluble in water.
(ii) It's solubility in hot water is less than that of cold water. Hence solubility decreases with increase in temperature.
(iii) It readily absorbs CO2 as used as a test for the gas.
(iv) it is used as a mortar.
[Mortar is a mixture of slaked lime (1 part) and sand (3 parts) made into paste with water. ]
Carbonates :
Sodium Carbonate:
Preparation :
(i) Leblanc Process :
NaCl + H2SO4(conc.) NaHSO4 + HCl
NaCl + NaHSO4 Na2SO4 + HCl
(Salt Cake)
Na2SO4 + 4C Na2S + 4CO
Na2S + CaCO3 Na2CO3 + CaS
(ii) Solvay Process :
NH3 + H2O + CO2 NH4HCO3
NaCl + NH4HCO3 NaHCO3 + NH4Cl
2NaHCO3 Na2CO3 + H2O + CO2
Properties :
(i) Anhydrous Na2CO3 is called as soda ash, which does not decompose on heating but melts at 852ºC
(ii) It forms number of hydrates.
Na2CO3.H2O Crystal carbonate
Na2CO3 moisture in air
Na2CO3.7H2O ________
Na2CO3.10H2O Washing soda
(iii) Na2CO3 absorbs CO2 yielding sparingly soluble sodium bicarbonate which can be calcined at 250º to get pure sodium carbonate.
Na2CO3 + H2O + CO2 2NaHCO3
(iv) It dissolved in acid with effervescence of CO2 and causticised by lime to give caustic soda.
Na2CO3 + HCl 2NaCl + H2O + CO2
Na2CO3 + Ca(OH)2 2NaOH + CaCO3
Uses : It is widely used in glass making as smelter.
Potassium Carbonate :
By leblance process, it can be prepared but by solvay process it cannot be prepared because KHCO3 is soluble in water.
Properties : It resembles with Na2CO3, m.p. is 900ºC but a mixture of Na2CO3 and K2CO3 melts at 712ºC.
Uses : It is used in glass manufacturing.
Calcium Carbonate :
It ocurs in nature as marble, limestone, chalk, coral, calcite etc. It is prepared by dissolving marble or limestone in HCl and removing iron and aluminium present, by precipitating with NH3 and then adding (NH4)2CO3 to the solution.
CaCl2 + (NH4)2CO3 CaCO3 + 2NH4Cl
Properties:
(i) It dissociates above 1000ºC as follows:
CaCO3 CaO + CO2
(ii) It dissolves in water containing CO2 forming Ca(HCO3)2 but is precipitated from the solution by boiling.
CaCO3 + H2O + CO Ca(HCO3)2
Magnesium carbonate :
It occurs in nature as magnesite, isomorphous with calcite. It is obtained as a white precipitated by adding sodium bicarbonate to a solution of a magnesium salt; but only basic carbonate, called magnesia alba, having the approximate composition MgCO3, Mg(OH)2, 3H2O is precipitated.
Properties : Same with CaCO3
Bicarbonates :
Sodium bicarbonates :
Preparation : By absorption of CO2 in Na2CO3 solution.
Na2CO3 + H2O + CO2 2NaHCO3
(> 100ºC sparingly soluble)
Uses : It is used in medicine and as baking powder.
Potassium bicarbonates :
Preparation : Same NaHCO3
Properties : Same with NaHCO3
But It is more alkaline and more soluble in water compared NaHCO3.
Magenisum bicarbonate :
MgCO3 + CO2 + H2O Mg(HCO3)2
Calcium bicarbonate :
CaCO3 + CO2 + H2O ⇔ Ca(HCO3)2
Chlorides :
Sodium Chloride : Prepared from brine containing 25% NaCl.
Properties:
(i) It is nonhygroscopic but the presence of MgCl2 in common salt renders it hygroscopic.
(ii) It is used to prepare freezing mixture in laboratory [Ice-common salt mixture is called freezing mixture and temperature goes down to - 23ºC.
(iii) For melting ice and snow on road.
Potassium Chloride : It is also occurs in nature as sylvyne (KCl) or carnalite (2KCl.MgCl2.6 H2O)
Uses : It is used as fertiliser.
Magnesium Chloride :
Preparation : By dissolving MgCO3 in dil. HCl
MgCO3 + 2HCl MgCl2 + H2O + CO2
Properties :
(i) It crystallises as hexahydrate. MgCl2.6H2O
(ii) It is deliquescent solid.
(iii) This hydrate undergoes hydrolysis as follows:
MgCl2.6H2O Mg(OH)Cl + HCl + 5H2O
Mg(OH)Cl MgO + HCl
** Hence, Anh. MgCl2 cannot be prepared by heating this hydrate.
** Because of this formation of HCl. Sea water cannot be used in marine boilers which corrodes the iron body.
(iv) Anhydrous MgCl2 can be prepared by heating a double salt like. MgCl2 . NH4Cl . 6 H2O as follows:
MgCl2.NH4Cl.6H2O MgCl2.NH4Cl
MgCl2 + NH3 + HCl
Sorel Cement : It is a mixture of MgO and MgCl2 (paste like) which set to hard mass on standing. This is used in dental filling, flooring etc.
Calcium chloroide :
(i) It is the by-product in solvay process.
(ii) It may also be prepared by dissolving the carbonate in HCl .
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Properties :
(i) It is deliquescent crystals.
(ii) It gets hydrolysed like MgCl2 hence anhydrous CaCl2 cannot be prepared.
CaCl2 + H2O CaO + 2HCl
Hence, anh CaCl2 is prepared by heating CaCl2.6H2O in a current of HCl (dry)
(iii) Anh. CaCl2 is used in drying gases and organic compounds but not NH3 or alcohol due to the formation of CaCl2.8NH3 and CaCl2.4C2H5OH.
Sodium sulphate :
Preparation :
It is formed in the 1st step of leblanc process by heating common salt with sulphuric acid.
2NaCl + H2SO4 → Na2SO4 + 2HCl
Thus the salt cake formed is crystallised out from its aqueous solution as Na2SO4.10H2O. This called as Glauber's salt.
** One interesting feature of the solubility of glauber's salt is: when crystallised at below 32.4ºC, then Na2SO4. 10H2O is obtained but above 32.4ºC, Na2SO4 (anh.) comes out.
Properties : It is reduced to Na2S when fused with carbon.
Na2SO4 + 4C → Na2S + 4CO
Uses : It is used in medicine.
Potassium Sulphate :
It occurs in stassfurt potash beds as schonite K2SO4. MgSO4.6H2O and Kainite, KCl.MgSO4.3H2O from which it is obtained by solution in water and crystallisation. It separates from the solution as anhcrystals whereas Na2SO4 comes as decahydrate.
Uses : It is used to prepare alumn.
Magnesium Sulphate :
Preparation :
(i) It is obtained by dissolving kieserite. MgSO4.H2O in boiling water and then crystallising the solution as a hepta hydrate. i.e. MgSO4. 7H2O. It is called as Epsom salt.
(ii) It is also obtained by dissolving magnesite in hot dil. H2SO4.
MgCO3 + H2SO4 → MgSO4 + H2O CO2
(iii) Or by dissolving dolomite (CaCO3, MgCO3) in hot dil. H2SO4 and removing the insoluble CaSO4 by filtration.
(iv) It is isomorphous with FeSO4.7H2O, ZnSO4.7H2O
Calcium Sulphate : It occurs as anhydrite CaSO4 and as the dihydrate CaSO4. 2H2O, gypsum, alabaster or satin-spar.
Properties :
(i) Gypsum (CaSO4.2H2O) 2CaSO4.H2O (Plaster of paris)
200oC
(anhydrous) CaSO4
Dead burnt. plaster
(ii) Solubility of CaSO4 at first increases upto a certain point and then decreases with rise of temperature.
(iii) Plaster of paris is used in mould making due to its porous body.
FAQs on S Block elements, Class 11, Chemistry Detailed Chapter Notes - JEE
| 1. What are S Block Elements? |  |
| 2. What are some important characteristics of S Block Elements? |  |
| 3. What are some common uses of S Block Elements? |  |
| 4. What is the difference between alkali metals and alkaline earth metals? |  |
| 5. Why are S Block Elements important in the context of JEE Chemistry? |  |




















