Change in States of Matter and Evaporation | Science Class 9 PDF Download
| Table of contents |

|
| Introduction |

|
| Can Matter Change its State? |

|
| Latent Heat (Hidden Heat) |

|
| Condensation |

|
| Freezing |

|
| Sublimation |

|
| Evaporation |

|
| Plasma |

|
| Bose-Einstein condensate (BEC) |

|
Introduction
- A substance may exist in three states of matter i.e., solid, liquid or gas, depending upon the conditions of temperature and pressure.
- By changing the conditions of temperature and pressure, all three states could be obtained (solid, liquid, gas). On heating, a solid changes into a liquid which on further heating changes into a gas.
Example: Water exists in all three states.
Solid: Ice, Liquid: Water, Gas: Water Vapor. - Ice is a solid state and may be melted to form water (liquid) which on further heating changes into steam (gas). These changes can also be reversed on cooling.
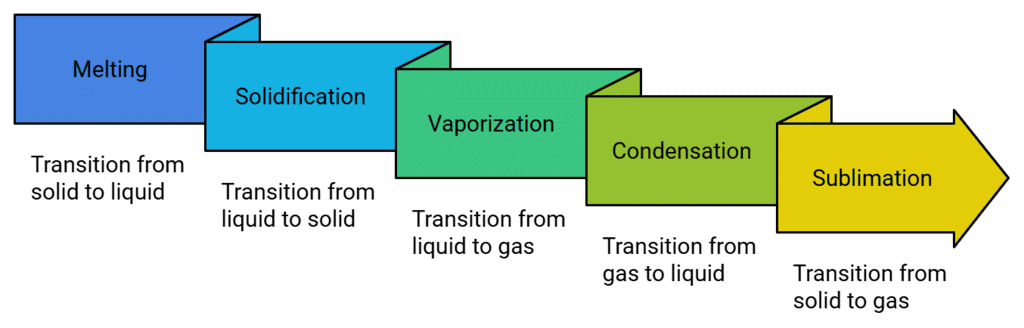 Phase Changing Processes
Phase Changing Processes
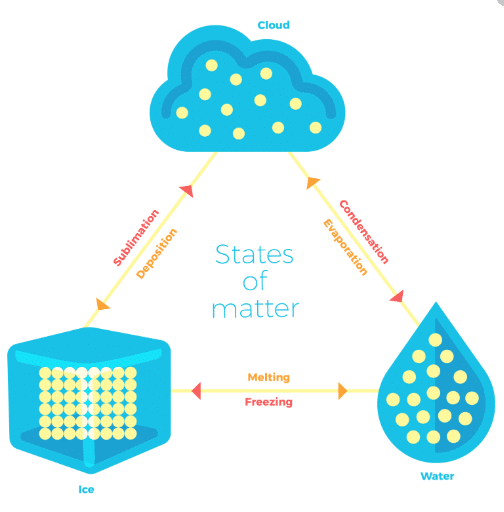
Can Matter Change its State?
Temperature and pressure are the two factors which decide whether a given substance would be in a solid, liquid or gaseous state.
1. Effect of Change of Temperature
Activity
The effect of temperature on three states of matter could be seen by performing the following activity.
- Procedure
(i) Take a piece of about 100 - 150 g of ice in a beaker.
(ii) Hang a thermometer in it so that its bulb is in contact with ice.
(iii) Start heating the beaker slowly on a low flame.
(iv) Note down the temperature when ice starts changing of water & ice has been converted to water.
(v) Record all observations for the conversion of solid ice into liquid water.
(vi) Now, place a glass rod in the beaker and slowly heat the beaker with constant stirring with help of a glass rod.
(vii) Note the temperature when water starts changing into water vapours.
(viii) Record all observations for the conversion of water in the liquid state to vapour state. - Observation: It is observed that as temperature increases, the ice starts changing into water. This change is called "Melting". The temperature remains same till all the ice changes into water. The thermometer shows 0°C until all the ice has melted. On further heating, the temperature starts rising. At 373 K (or 100°C), water starts boiling. As the water continues to boil, the temperature remains almost constant.
- Conclusion of the above activity
This experiment demonstrates that we can change the physical state of matter by heating (Solid → Liquid → Gas).
Solid to Liquid Change (Melting)
- Ice is solid. In solids, the particles are tightly packed together. When we heat a solid, its particles become more energetic and kinetic energy of the particles increases. Due to the increase in kinetic energy, the particles start vibrating more strongly with greater speed.
- The energy supplied by heat overcomes the intermolecular forces of attraction between the particles. As a result, the particles leave their mean position and break away from each other. After this, solid melts and a liquid is formed.
- The temperature at which solid melts to become a liquid at atmospheric pressure is called its Melting point(or fusion).
- The process of melting is also called "Fusion". The melting point of ice is 0°C. It may also be written as 273.16 K or 273 K.
- The temperature is represented in Celsius scale as °C. Nowadays, it is expressed on Kelvin scale (K).
- Conversion of temperature on Celsius scale to the Kelvin scale
Example:
0°C = 0 + 273 = 273 K
100°C = 100 + 273 = 373 K - Conversion of temperature on Kelvin scale to Celsius scale
Example:
-373 K = 373 - 273 = 100°C
273 K = (273 - 273) = 0°C
Liquid to Gas Change (Boiling or Vapourization)
- When we continue heating the water, at 100°C water molecules again break the force of attraction and become vapour. Here, 100°C acts as an equilibrium point, and any energy beyond this is utilized to break the force of attraction. So the temperature also remains the same, i.e. 100°C. We call this latent heat as latent heat of evaporation.
- The temperature at which a liquid changes into a gas or vapour at the atmospheric pressure is called its boiling point.
Example: For water, the boiling point is 100°C or 373 K. The particles in steam i.e., water vapour at 373 K have more energy than water at the same temperature. Reason: This is because the particle in steam has absorbed extra energy in the form of latent heat of vaporization. - The boiling point of a liquid also indicates the strength of the intermolecular force of attraction between particles. Volatile liquids such as alcohol, petrol, and acetone have very weak intermolecular forces. Therefore, they boil at a low temperature. On the other hand, water has stronger inter-molecular forces of attraction and therefore, it boils at a higher temperature. When steam is cooled, it condenses to water & when water is cooled, it changes to ice.
Example: If the boiling point of water is 100°C (373 K), then the liquefaction point of steam is 100°C (373 K).
2. Effect of Change of Pressure
- Gases are compressible because of applying pressure, the space between the gaseous particles decreases. Therefore gases can be compressed easily.
- By applying pressure and reducing temperature, the gases can be converted into liquids i.e. gases will be liquefied.
- This process of conversion of a gas into a liquid by increasing pressure or decreasing temperature is called liquefaction.
- Solid carbon dioxide is also called dry ice. Solid CO2 gets converted into gaseous state directly on decreasing pressure to 1 atmosphere without coming into the liquid state.
Thus, we can conclude that temperature and pressure determine the state of a substance solid, liquid or gaseous.
Latent Heat (Hidden Heat)
It is observed that the temperature of the system does not change after melting point is achieved until all the ice melts, though we continue to heat the beaker.- This is happening because the heat supplied is used up in changing the state by breaking the intermolecular forces of attraction which holds them in the solid state. As a result, there is no change in temperature until all the ice melts. This energy required to change a solid into liquid is called "latent heat". The word "latent" means "hidden" because this energy is hidden into the contents of the beaker.
- Latent heat is of two types:
(i) Latent heat of fusion.
(ii) Latent heat of vaporization.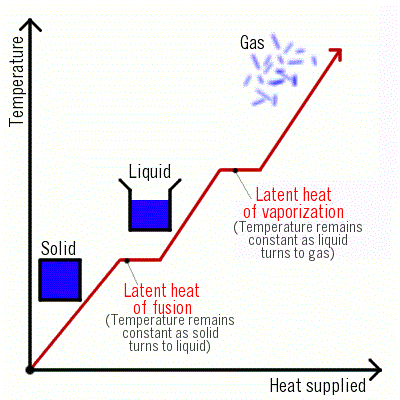
Latent Heat of Fusion
- Latent heat of fusion is defined as the amount of heat energy required to change 1 kg of a solid into a liquid at atmospheric pressure without any change in temperature at its melting point.
- The latent heat of the fusion of ice is 3.34 × 105 J/Kg.
- The numerical value of melting point and freezing point is the same.
Example: If the melting point of ice is 0°C (273 K), then the freezing point of water is 0°C (273 K).
Latent Heat of Vaporization
- The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 kilogram of the liquid (at its boiling point) to vapour or gas, without any change in temperature.
- The latent heat of vaporization of water is 22.5 × 105 joules per kilogram (or 22.5 × 105 J/kg).
Condensation
 Formation of Water droplets on the surface of a cold bottle due to the condensation of water vapour present in the air.
Formation of Water droplets on the surface of a cold bottle due to the condensation of water vapour present in the air.
- The process of changing a gas (or vapour) to a liquid by cooling is called condensation where gas is cooled enough.
- So, when steam (or water vapour) changes into water on cooling, it is called condensation of steam (or condensation of water vapour).
- It is the reverse of vaporization. (Boiling)
Freezing
- The process of changing a liquid (solidification) into a solid by cooling is called freezing.
- When a liquid is cooled, its particles lose energy due to which they move slowly.
- If the liquid is cooled enough (upto freezing point) its each particle stops moving and vibrates about a fixed position. At this stage, the liquid freezes and becomes a solid.
- Freezing is the reverse of melting.
Sublimation
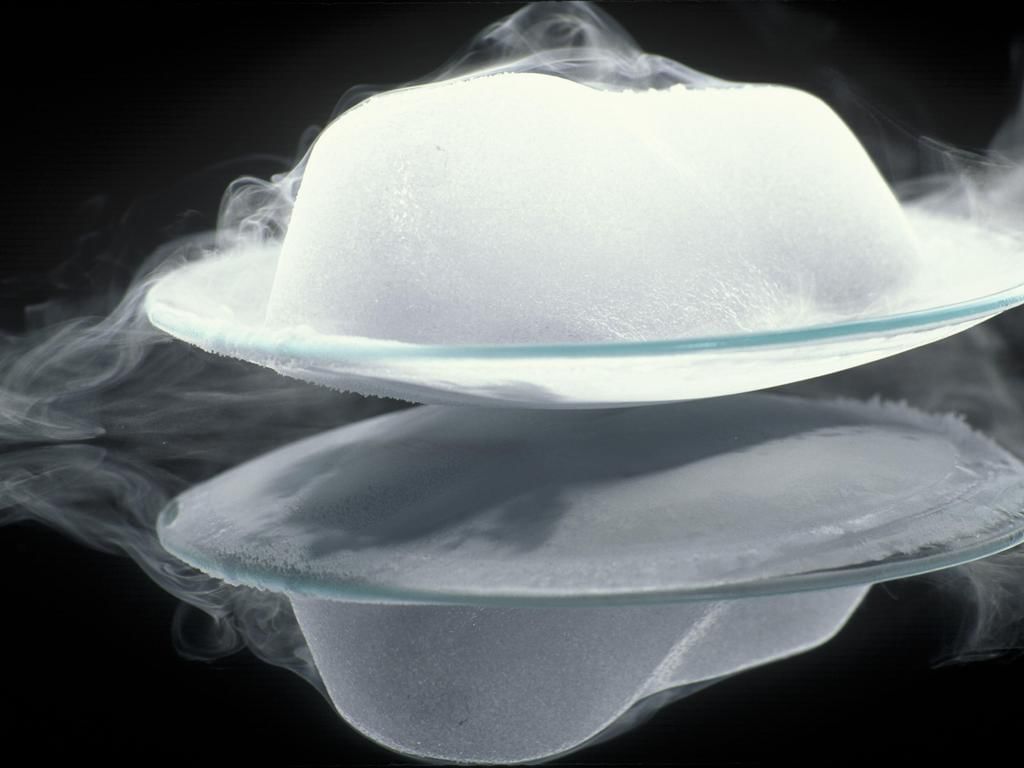 Sublimation
Sublimation- The process due to which a solid directly changes into the gaseous state on heating, without changing first into liquid state and the gaseous state, directly changes into the solid state on cooling, is known as "Sublimation".
Examples: Ammonium chloride, Camphor, Iodine, Naphthalene, Solid carbon dioxide or (dry Ice). - Sublime: A gaseous form, directly formed from a solid on heating, is known as sublime.
- Sublimate: A solid-state of matter formed directly from its gaseous state on cooling is called sublimate.
- To understand the sublimation process we can do an activity:
> Take some camphor or ammonium chloride.
> Powder it and put in a china dish.
> Place an inverted funnel over the china dish.
> Put a cotton plug on the stem of the funnel, as shown in the Figure below.
> Heat the china dish slowly.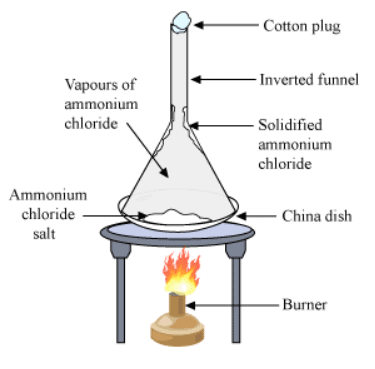 Sublimation
Sublimation - Observation: Heating camphor or ammonium chloride results in its vapour formation. These vapour cool at the upper part of the beaker and deposit there.
- Explanation: Solid state is directly converted into the gaseous state. This experiment shows the sublimation process. Cotton plug on the beaker prevents the escape of the formed vapour. In the upper part of the beaker, the temperature is lesser. As a result, the vapours cool down and deposit as solids on the neck of the beaker.
- This process of conversion of gas to solid is inverse of sublimation and we call this process Deposition.
Evaporation
- The process of a liquid changing into vapour (or gas) even below its boiling point is called evaporation.
- Evaporation of a liquid can take place even at room temperature, though it is faster at higher temperatures. It is the surface phenomenon because it occurs at the surface of a liquid only. Whatever be the temperature at which evaporation takes place, the latent heat of vaporization must be supplied whenever a liquid changes into vapour (or gas).
 |
Download the notes
Change in States of Matter and Evaporation
|
Download as PDF |
Explanation about Evaporation
- Some particles in a liquid always have more kinetic energy than the others. So, even when a liquid is well below its boiling point, some of its particles have enough energy to break the forces of attraction between the particles and escape from the surface of the liquid in the form of vapour (or gas).
- Thus, the fast-moving particles (or molecules) of a liquid are constantly escaping from the liquid to form vapour (or gas).
- Examples:
(i) Water in ponds changes from liquid to vapour without reaching the boiling point.
(ii) Water when left uncovered slowly changes into vapours.
(iii) When we put wet clothes for drying, the water from the clothes goes to the atmosphere.
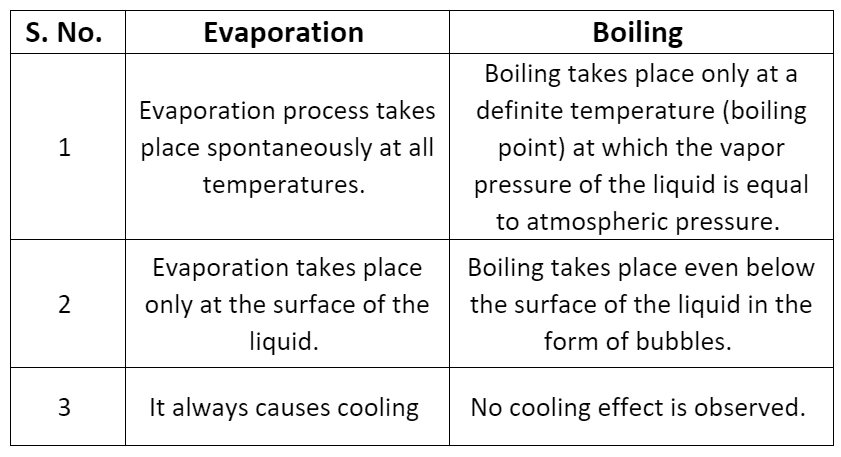
Differences between Evaporation and Boiling
Factors Affecting Evaporation
There are five factors which affect the rate of evaporation:
- Nature of liquid: Different liquids have different rates of evaporation. A liquid having weaker interparticle attractive forces evaporates at a faster rate because less energy is required to overcome the attractive forces.
Example: Acetone evaporates faster than water. - The surface area of the liquid: The evaporation depends upon the surface area. If the surface area is increased, the rate of evaporation increases because the high-energy particles from the liquid can go into the gas phase only through the surface.
Example:
(i) The rate of evaporation increases when we put kerosene or petrol in an open china dish than in a test tube.
(ii) Clothes dry faster when they are well spread because the surface area for evaporation increases. - Temperature: Rate of evaporation increases with increase in temperature. This is because with the increase in temperature number of particles gets enough kinetic energy to go into the vapour state (or gaseous state).
Example: Clothes dry faster in summers than in winters. - Humidity in the air: The air around us contains water vapour or moisture. The amount of water present in the air is referred to as humidity. The air cannot hold more than a definite amount of water vapour at a given temperature. If the humidity is more, the rate of vaporization decreases. The rate of evaporation is more if the air is dry.
Example: Clothes do not dry easily during the rainy season because the rate of evaporation is less due to high moisture content (humidity) in the air. - Wind speed: The rate of evaporation also increases with increase in speed of the wind. This is because with increase in speed of wind, the particles of water vapour move away with wind resulting in a decrease in the amount of vapour in the atmosphere.
Example:
(i) Clothes dry faster on a windy day.
(ii) In a desert cooler an exhaust fan sucks the moist air from the cooler chamber which results in a greater rate of evaporation of water and hence greater cooling.
How does Evaporation cause Cooling?
- During evaporation, cooling is always caused. This is because evaporation is a phenomenon in which only the high energy particles leave the liquid surface. As a result, the particles having low energy are left behind. Therefore, the average molecular energy of the remaining particles left in the liquid state is lowered. As a result, there is a decrease in temperature on the part of the liquid that is left. Thus evaporation causes cooling.
- Examples:
(i) When we pour some acetone on our palm, we feel cold. This is because the particles gain energy from our palm or surroundings and leave the palm feeling cool.
(ii) We sprinkle water on the root of the open ground after a sunny hot day. This cools the roof or open ground. This is because the large latent heat of vaporization of water helps to cool the hot surface.
Some other examples of Evaporation
- We should wear cotton clothes in hot summer days to stay cool and comfortable: This can be explained as follows. We get a lot of sweat on our bodies on hot summer days. Cotton is a good absorber of water, so it absorbs the sweat from our body and exposes it to the air for evaporation. The evaporation of this sweat cools our body. Synthetic clothes (made of polyester etc.) do not absorb a lot of sweat, so they fail to keep our body cool in summer.
- We see water droplets on the outer surface of a glass containing ice-cold water: Take some ice-cold water in a glass. Soon we will see water droplets on the outer surface of the glass.
 Water Droplets
Water Droplets - The water vapour present in the air, on coming in contact with the cold glass of water loses energy and gets converted to the liquid state, which we see as water droplets.
- Water keeps cool in the earthen pot (matki) during summer: When the water oozes out of the pores of an earthen pot, during hot summer, it evaporates rapidly. As the cooling is caused by evaporation, therefore, the temperature of water within the pot falls and hence it becomes cool.
 Earthen Pot
Earthen Pot
- Rapid cooling of hot tea: If tea is too hot to sip, we pour it into the saucer. In doing so, we increase the surface area and the rate of evaporation. This, in turn, causes cooling and the tea attains the desired temperature for sipping.
- A wet handkerchief is placed on the forehead of a person suffering from a high fever: The logic behind placing wet cloth is that as the water from the wet cloth evaporates, it takes heat from the skull and the brain within it. This, in turn, lowers the temperature of the brain and protects it from any damage due to high temperature.
- We often sprinkle water on the road in summer: The water evaporates rapidly from the hot surface of the road, thereby taking heat away from it. Thus, the road becomes cool.
Plasma
- Plasma is a mixture of free electrons and ions.
- Plasma is considered the fourth state of matter.
- Plasma occurs naturally in the stars (including the sun). Inside the stars, the temperature is so high that the atoms break up. Some of the electrons break away from the atoms converting the rest of the atoms into electrically charged particles called ions. This mixture of free electrons and ions in a star is called plasma.
- The sun and other stars glow because of the presence of plasma in them.
- Plasma can also be made on the earth by passing "electricity through gases at very low pressure taken in a glass tube (called discharge tube).
- Plasma makes a fluorescent tube (or neon sign bulb) glow.
Bose-Einstein condensate (BEC)
In 1920, an Indian scientist "Satyendra Nath Bose" did some calculations for the fifth state of matter. On the basis of these calculations, Albert Einstein predicted the existence of a new state of matter called Bose-Einstein condensate (BEC).
The fifth state of matter called Bose-Einstein condensate was finally achieved by three scientists Cornell, Ketterle and Wieman of USA by cooling a gas of extremely low density (about one hundred-thousandth the density of normal air) to super-low temperatures.
|
84 videos|384 docs|61 tests
|
FAQs on Change in States of Matter and Evaporation - Science Class 9
| 1. What are the different states of matter and how can they change? |  |
| 2. What is latent heat and why is it important in state changes? |  |
| 3. How does condensation occur and what factors influence it? |  |
| 4. What is the process of sublimation and where can we observe it in nature? |  |
| 5. What is a Bose-Einstein condensate (BEC) and how is it formed? |  |


















