Important Compounds of Calcium | Chemistry for JEE Main & Advanced PDF Download
Calcium Oxide - CaO
Calcium oxide, commonly known as lime, is a chemical compound with the formula CaO.
Calcium oxide, also known as quicklime, is an alkaline substance that has been in use since the medieval age. It is believed that quicklime is one of the oldest chemicals known to the human race. It can also be referred to as burnt lime or lime. Calcium oxide has a medium viscosity and a high surface tension, plus a high to intermediate expansion and contraction rate. This material isn’t volatile at ceramic temperatures. Calcium oxide has a moderate effect on colour, except in large amounts when it may have a bleaching effect on iron oxide. It also exists in the colour of kaki/tomato reds.
Calcium oxide has a medium viscosity and a high surface tension, plus a high to intermediate expansion and contraction rate. This material isn’t volatile at ceramic temperatures. Calcium oxide has a moderate effect on colour, except in large amounts when it may have a bleaching effect on iron oxide. It also exists in the colour of kaki/tomato reds.
Preparation of Calcium Oxide
- Calcium oxide can be produced by thermal decomposition of materials like limestone or seashells that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln.
- The process that is used to prepare burnt lime is known as calcination. It is a process that starts with thermally decomposing the reactants at high temperatures but ensuring that the temperature is kept well below the melting point.
- Calcium carbonate undergoes calcination at temperatures ranging between 1070ºC-1270ºC. These reactions are usually held in a rotary kiln. The products formed as a result of the reaction are burnt lime and carbon dioxide.
The carbon dioxide that is formed is immediately removed so that the reaction is preceded until the completion of the process in accordance with Le-Chatelier’s principle.
CaCO3 → CaO + CO2
This reaction is reversible and exothermic in nature in the forward direction.
Structure of CaO Molecules
Calcium oxide molecules contain one calcium cation (which holds a charge of +2) and one oxygen anion (which holds a charge of -2). The structure of calcium oxide is illustrated below.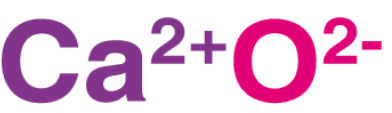 Thus, it can be understood that calcium oxide is an ionic compound featuring an ionic bond between calcium and oxygen.
Thus, it can be understood that calcium oxide is an ionic compound featuring an ionic bond between calcium and oxygen.
Lime Water Formula
The formula for lime water is Ca(OH)2 and the chemical name for lime water is calcium hydroxide. When water is added to lime calcium hydroxide Ca(OH)2 is formed according to the following reaction.
CaO + H2O → Ca(OH)2
This reaction is strongly exothermic and takes place vigorously with the formation of clouds of steam.
What is the Difference Between Quicklime and Lime Water?
The chemical formula of lime or quicklime is CaO. The chemical name of lime is calcium oxide. On the other hand, the chemical formula of limewater is Ca(OH)2 and the chemical name of this substance is calcium hydroxide.
Properties of Calcium Oxide
- Quick lime is an amorphous white solid with a high melting point of 2600°
- It is a very stable compound and withstands high temperatures.
- In the presence of water, it forms slaked lime. This process is called the slaking of lime.
CaO + H2O → Ca (OH)2.
- It is an oxide that is basic in nature and forms salts when it comes in contact with an acid.
- This compound crystallizes in a cubic crystal lattice.
- The standard molar entropy associated with calcium oxide corresponds to 40 joules per mole kelvin.
- This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius.
CaO + H2SO4 → CaSO4 + H2O
Uses of Calcium Oxide
- It is extensively used for medicinal purposes and insecticides.
- It finds its application in the manufacturing of cement, paper, and high-grade steel.
- Lime is used as a reagent in laboratories for dehydration, precipitation reaction, etc.
- It is the cheapest alkali available which is an important ingredient in the manufacturing of caustic soda.
- Calcium is essential to animal life as the constituent of bones, shells, and teeth. The most common of the calcium compounds are calcium carbonate which the potter uses as a source of calcium oxide for glazes.
Important Safety Tips
- There are few things that users should keep in mind regarding Calcium Oxides.
- The reaction between quicklime and water is usually vigorous.
- Quicklime can cause severe irritation, especially when inhaled or if it comes in contact with wet skin or eyes.
- Some of the effects of inhalation include sneezing, coughing, or laboured breathing.
- Additionally, it can also result in abdominal pain, nausea burns with perforation of the nasal septum, and vomiting.
- When quicklime reacts with water it can release enough heat to even ignite combustible materials.
Calcium Hydroxide - Ca(OH)2
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca(OH)2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca(OH)2 has a colourless appearance in its crystalline form.
The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide (also known as quick lime).
The chemical reaction between sodium hydroxide and calcium chloride dissolved in water (aqueous CaCl2) also yields this compound. The structure of a Ca(OH)2 molecule is illustrated below.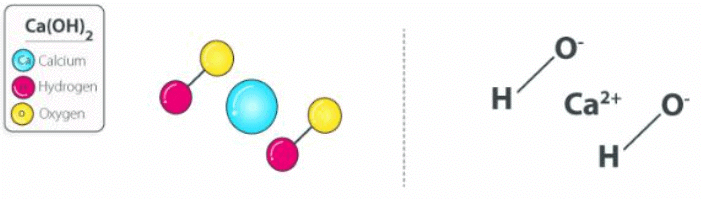 Calcium Hydroxide Structure
Calcium Hydroxide Structure
Molecules of calcium hydroxide are held together via ionic bonds between the calcium ion (Ca2+) and two hydroxide ions (OH–). Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca(OH)2 can lead to lung damage and even blindness.
Properties of Calcium Hydroxide
Some important properties of calcium hydroxide are tabulated below.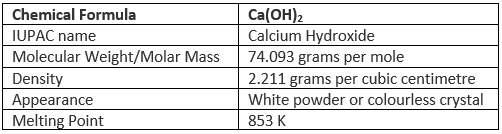
Physical Properties
- Ca(OH)2 has a hexagonal crystal structure.
- It is not very soluble in water and its solubility reduces with an increase in temperature. For example, its solubility at 0ºC is 1.89 g/L and its solubility is 1.73 g/L at 20º.
- At temperatures approaching its melting point, this compound tends to lose water and decompose.
- The solubility product (Ksp) of calcium hydroxide is 5.5 * 10-6.
Chemical Properties
- Ca(OH)2 is quite soluble in glycerol and acids, but only slightly soluble in water. When dissolved in water to a saturation point, it yields a solution which acts as a moderate base (called limewater).
- Limewater reacts with acids and forms salts.
- The saturated solution of calcium hydroxide in water also reacts with and dissolves metals such as aluminum.
- It reacts with carbon dioxide to form calcium carbonate (CaCO3). This reaction is commonly referred to as carbonatation.
Uses of Calcium Hydroxide
- In the process of sewage treatment, calcium hydroxide is used as a clarifying agent or as a flocculant.
- Ca(OH)2 is used in the paper industry during the Kraft process of converting wood into wood pulp.
- It is a very important compound in the preparation of ammonia.
- This compound is also used as a pH modifier due to its basicity.
- The pickling of cucumbers is generally done with the help of Ca(OH)2.
- The production of many plastics involves the use of calcium hydroxide as an ingredient.
- It is also used in pesticides, hair care products, and the manufacture of ebonite.
- In root canal procedures, this compound is used to fill the cavities in the human teeth.
- Sugar beets and sugarcane are processed via carbonation, which involves the used of Ca(OH)2.
- Calcium hydroxide is used in the leather industry to separate the fur/hair from the animal hide.
Calcium Carbonate
Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO3.
Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms, where the use of chalk (a form of CaCO3) is found. It is found in the earth’s crust. It is also found in many forms such as marble, limestone, etc. Although they are available in various forms they are chemically similar and only differ physically. They are also referred to as calcite.
Calcium carbonate is a non-toxic and odourless compound commonly found as a white mineral which occurs naturally in chalks, limestones and marbles. Calcium Carbonate
Calcium Carbonate
Commercial Production of Calcium Carbonate
Calcium carbonate is produced commercially in two different grades. Both grades compete industrially based primarily on particle size and the characteristics imparted to a product.
- Ground Calcium Carbonate: Produced via extraction and processing of naturally occurring deposits. GCC crystal shape is irregularly rhombohedral and has a broader size distribution.
- Precipitated Calcium Carbonate: Produced via chemical precipitation via a carbocation process or as a by-product of some bulk chemical processes. PCC crystal shape depends on the product and the product and the particles are more uniform and regular with a narrow size distribution.
PCC has smaller particles has a higher purity is less abrasive and tends to have higher brightness than GCC.
Calcium Carbonate Structure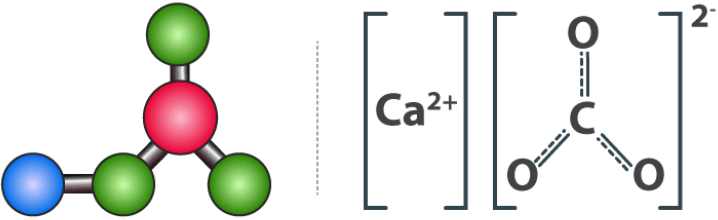 Structure of Calcium Carbonate
Structure of Calcium Carbonate
Calcium Carbonate Formula
- It is a chemical compound with the chemical formula CaCO3.
- It is a white insoluble powder-like substance which occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc.
- Medicinally, it is used as an antacid or as a calcium supplement. It is also used as fillers in cosmetics. It is added to swimming pools as a disinfectant agent and as pH corrector.
- It finds extensive usage in the manufacturing industry as a building material (marble), ingredient for quick lime and cement.
Preparation of CaCO3
- CaCO3 is obtained by using carbon dioxide and slaked lime as raw materials. When carbon dioxide is passed through slaked lime, calcite is obtained.
Another method to obtain calcite is by adding sodium carbonate to calcium chloride.
Ca (OH)2 + CO2 → CaCO3 + H2O
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
When carbon dioxide is passed in excess it leads to the formation of calcium hydrogen-carbonate. - On a large scale, it is prepared by passing carbon dioxide gas through calcium hydroxide (slaked lime). However, if carbon dioxide is passed in excess, it forms the soluble calcium hydrogen-carbonate.
Ca(OH)2 + CO2 → CaCO3 + H2O
Properties of CaCO3
- It is a fluffy powder.
- It decomposes to give carbon dioxide when heated up to 1200K.
- When it reacts with dilute acid, it liberates carbon dioxide as a by-product.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2 - At 1200K, calcium carbonate decomposes to give carbon dioxide and calcium oxide.
CaCO3 → CaO + CO2 - On reacting with dilute acids, calcium carbonate gives carbon dioxide.
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Application of Calcium Carbonate
- Calcium carbonate is largely employed in the pulp and paper industry. It can be used as a filter and pigment, making possible the production of a whiter, higher quality pigment than other minerals.
- In the construction industry, calcium carbonate is used as a filler in concrete, increasing its durability and appearance and to purify metals for use in construction applications.
- Another application of calcium carbonate is in fertilizers to provide calcium to plants and pH stabilization of the soil.
- Calcium carbonate can also be an additive to food products for livestock animals and humans and as a supplement in vitamins.
- In water and sewer treatment plants, calcium carbonate is employed in the removal of acidity and impurities.
Calcium Carbonate Uses
The list of uses of calcium carbonate is given below:
- It plays an important role in construction, be it as a building material (marble) or as an ingredient in cement.
- It is used in medicinal industries which manufacture antacids, tablets which are made of base materials etc.
- It is used as calcium supplements.
- It is used in the manufacture of paints, paper, plastics, etc.
 |
Download the notes
Important Compounds of Calcium
|
Download as PDF |
Calcium Sulphate - CaSO4
Calcium is an essential metal for living organisms. Due to its chemical properties, it finds usage in the preparation of alloys as a deoxidizer, desulfurizer and decarbonizer. However, it is not just the metal itself, a number of calcium compounds are also crucial to several industries and are prepared on a large scale.
Some of them are calcium carbonate, calcium oxide (quick lime), calcium hydroxide, calcium sulphate, calcium chloride, etc. Calcium Sulphate - CaSO4
Calcium Sulphate - CaSO4
What is Calcium Sulphate?
- It is a calcium compound with formula CaSO4 and its related hydrates.
- Its forms are white solids, barely soluble in water.
- It’s two common hydrates are Plaster of Paris and Gypsum.
- Plaster of Paris is a Hemi-hydrate of calcium sulphate which is obtained by heating gypsum to 393 K.

- If it is heated above 393 K, it forms anhydrite, also known as ‘dead burnt plaster’, which will again form gypsum on mixing with water.
- It is used in building industries, for the construction of false ceilings, like plasters, etc.
- In art, it is used for making statues and burial sites.
- In medicine, it is used indentures.
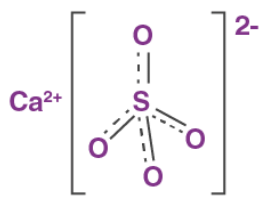
Hydration States
- CaSO4 (anhydrite): the anhydrous state.
- CaSO4 · 2 H2O (gypsum): dihydrate.
- CaSO4 · 0.5 H2O (bassanite): hemihydrate, also known as plaster of Paris. Specific hemihydrates are sometimes distinguished: alpha-hemihydrate and beta-hemihydrate.
Uses of Calcium Sulphate
- Its major use is in the manufacture of Plaster of Paris. Due to its ability to be moulded in the paste form on applying water to the powdered form of Calcium Sulphate.
- Calcium Sulphate is used in tofu as a coagulant.
- The compound was used to make sulphuric acid before the 1970s.
- Used as a moisture indicator.
|
353 videos|587 docs|309 tests
|
FAQs on Important Compounds of Calcium - Chemistry for JEE Main & Advanced
| 1. What are some important compounds of calcium? |  |
| 2. What is the significance of calcium carbonate? |  |
| 3. How is calcium chloride used in everyday life? |  |
| 4. What is the role of calcium hydroxide in agriculture? |  |
| 5. What is the importance of calcium phosphate in the human body? |  |























