Colloids & Its Classification | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| What is Colloid? |

|
| Classification of Colloids |

|
| (i) Based on Interaction between Dispersed Phase and Dispersion Medium |

|
| (ii) Based on the type of particles of the Dispersed Phase |

|
What is Colloid?
A colloid is primarily a heterogeneous mixture in which the minute particles of one substance are dispersed in another substance, called the dispersion medium.
The minute particles here are 1 to 1000 nanometers in diameter but they still remain suspended and do not settle at the bottom of the mixture. They are visible under an optical or an electron (smaller particles) microscope.
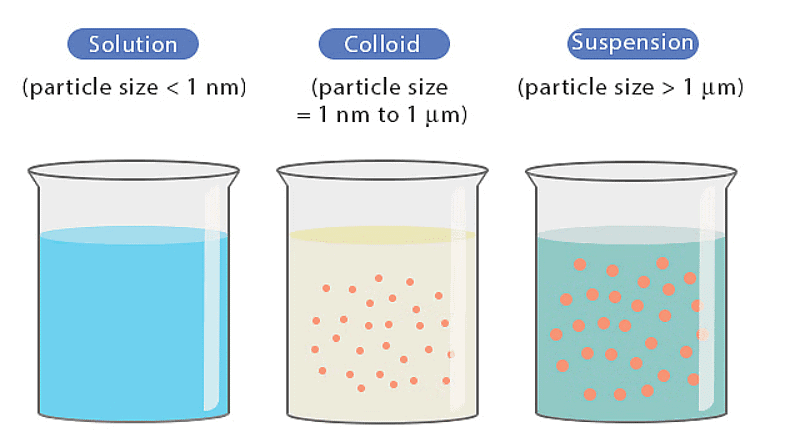
The distinction between True Solution, Colloidal Solution & Suspension:
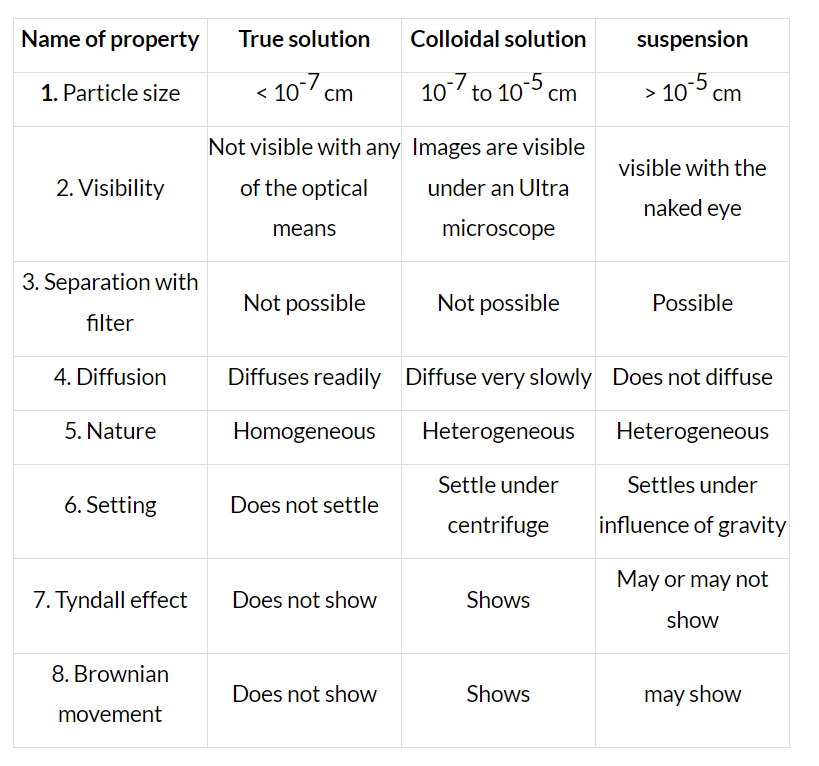
Dispersed Phase and Dispersion Medium
A colloidal system is heterogeneous in character. It consists of two phases, namely a dispersed phase and a dispersion medium.
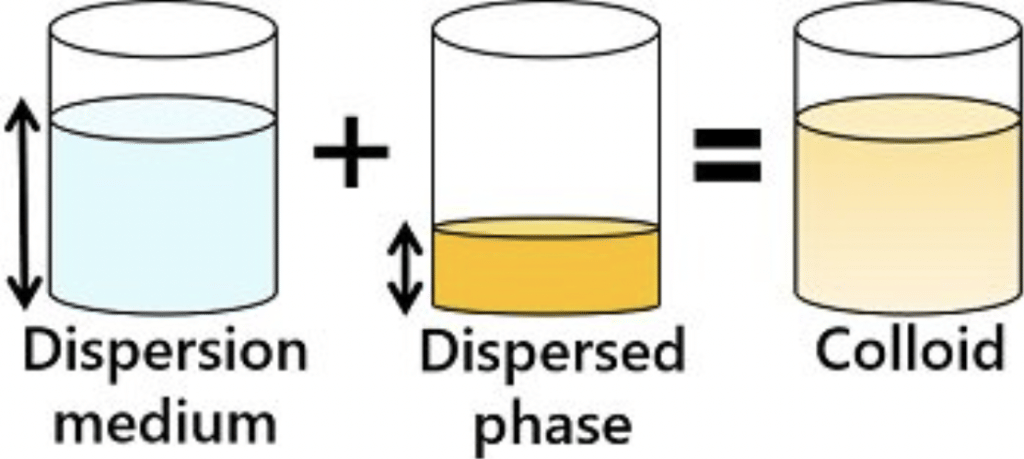
(a) Dispersed Phase (DP): It is the component present in small proportion and is just like a solute in a true solution. For example, in the colloidal state of sulphur in the water, the former acts as a dispersed phase.
(b) Dispersion Medium (DM): It is normally the component present in excess and is just like a solvent in a solution.
The particles of the dispersed phase are scattered in the dispersion medium in a colloidal system.
Classification of Colloids
Colloids can be classified in a number of ways based upon some of their important characteristics.
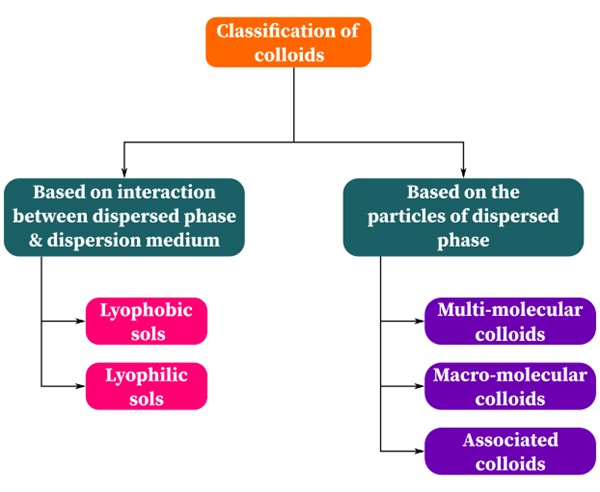
(i) Based on Interaction between Dispersed Phase and Dispersion Medium
On the basis of the affinity or interaction between the dispersed phase and the dispersion medium, the colloids may be classified into two types :
1. Lyophobic colloids:
- The colloidal system in which the dispersed phase has no affinity for the dispersion medium are called lyophobic (solvent hating) colloids.
- They are easily precipitated (or coagulated) on the addition of small amounts of the electrolyte, by heating or by shaking. They are less stable and irreversible.
- When the dispersion medium is water, these are known as hydrophobic colloids. Examples of lyophobic colloids include sols of metals and their insoluble compounds like sulphides and oxides.
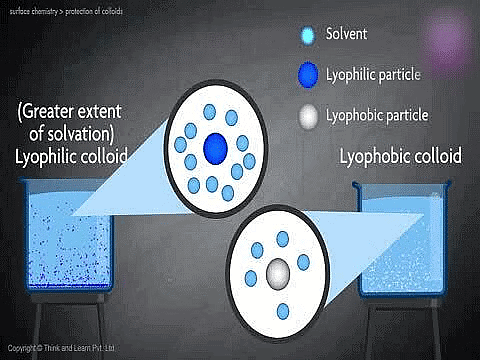
2. Lyophilic Colloids:
- The colloidal system in which the particle of dispersed phase has great affinity for the dispersion medium, are called lyophilic (solvent-loving) colloids.
- In such colloids, the dispersed phase does not get easily precipitated and the sols are more stable. Such colloidal systems, even if precipitated, may be reconverted to the colloidal state by simply agitating them with the dispersion medium. Hence lyophilic colloids are reversible.
- When the dispersion medium is water, these are called hydrophilic colloids. Some common examples of lyophilic colloids are gum, gelatin, starch, rubber, proteins, etc.
Difference between Lyophilic and Lyophobic Sols
| Property | Lyophilic/ hydrophilic sols | Lyophobic/ hydrophobic sols | |
1. | Nature | Reversible | Irreversible |
2. | Preparation | They are prepared very easily by shaking or warming the substance with dispersion medium. They do not required any electrolyte or stabilization. | They are difficult to prepare, Special methods are used. Addition of stabiliser is essential for their stability. |
3. | Stability | They are very stable and are not easily coagulated by electrolytes. | They are generally unstable and get easily coagulated on addition of electrolytes. |
4. | Charge | Particles carry no or very little charge depending upon the pH of the medium. | Colloidal particles have characteristic charge (positive or negative) |
5. | Viscosity | Viscosity is much higher than that of the medium. | Viscosity is nearly the same as that of the medium |
6. | Surface Tension | Surface tension is usually less than that of the medium. | Surface tension is nearly the same as that of the medium. |
7. | Migration in electric field | The particles may or may not migrate in an electric field. | The colloidal particles migrate either towards cathode or anode in an electric field |
8. | Solvation | Particles are heavily solvated. | Particles are not solvated. |
9. | Visibility | The particles cannot be seen under ultra microscope. | The particles though invisible, can be seen under ultra microscope. |
10. | Tyndall effect | Less distinct. | More distinct. |
11. | Action of electrolyte | Large amount of electrolyte is required to cause coagulation. | Small amount of electrolyte is sufficient to cause coagulation. |
12. | Examples | Mostly organic substances e.g. starch, gums, proteins, gelatin, rubber etc. | Generally inorganic substance e.g., metal sols, sulphides and oxides sols. |
(ii) Based on the type of particles of the Dispersed Phase
Depending upon the molecular size, the colloidal system has been classified into three classes :
- Multimolecular colloids: The multimolecular colloidal particles consists of an aggregate of atoms of small molecules with diameters less than 10-9 m or 1 nm.
For example, a sol. of gold contains particles of various sizes having several atoms. A sol. of sulphur consists of particles containing a thousand or so S2 molecules. These particles are held together by Vander Waal's forces. These are usually lyophobic sols for example gold sol. - Macromolecular colloids: The macromolecular colloidal particles themselves are large molecules. They have very high molecular weights varying from thousands to millions. These substances are generally polymers. Naturally occurring macromolecules are such as starch, cellulose, and proteins. Artificial macromolecules are such as polyethylene, nylon, polysyrene, dacron, synthetic rubber, plastics, etc. The size of these molecules is comparable to those of colloidal particles and therefore, their dispersion known as macromolecular colloids. Their dispersion also resembles true solutions in some respects. For example - Starch, cellulose, proteins, and enzymes.
- Associated colloids or micelles : These colloids behave as normal electrolytes at low concentrations but colloids at higher concentrations. This is because, at higher concentrations, they form aggregated (associated) particles called miscelles. Soap and synthetic detergents are examples of associated colloids. They furnish ions that may have colloidal dimensions.
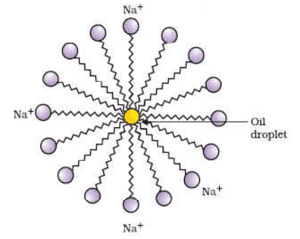 Associated Colloids(Micelle Formation)RCOONa -> RCOO— Na
Associated Colloids(Micelle Formation)RCOONa -> RCOO— Na
Sodium Stearate soap (R = C17H35)
The long-chain RCOO— ions associate or aggregate at higher concentrations and form miscelles and behave as colloids. They may contain 100 or more molecules.
Sodium stearate C17H35COONa is an example of an associated colloid. In water, it gives Na and sterate, C17H35COO— ions. These ions associate to form miscelles of colloidal size.
Colloids that behave as normal electrolytes at low concentration, but exhibit colloidal properties at higher concentration due to the formation of aggregated particles called micelles are referred to as associated colloids. The micelles are formed by the association of dispersed particles above a certain concentration and a certain minimum concentration is required for the process of aggregation to take place. The minimum concentration required for micelle formation is called micellisation concentration (CMC) and its value depends upon the nature of the dispersed phase. For soaps, CMC is 10-3 - 10-4 M.
Mechanism of Micelle Formation
Micelles are formed by surface-active molecules called surfactants such as soaps and detergents. These molecules have the lyophilic group at one end and a lyphobic group at the other end. Let us take the example of a soap (say sodium oleate, C17H33COO—Na ). The long hydrocarbon part of oleate radical (C17H33 -) is lyophobic end while COO— part is the lyophilic end. When the concentration of the solution is below its CMC, sodium oleate behaves like a normal electrolyte and ionizes to give C17H33COO— and Na ions. When the concentration exceeds CMC, the lyophobic part starts receding away from the solvent and tends to approach each other. However, the polar COO— ends tend to interact with the solvent (water). This finally leads to the formation of bigger molecules having the dimensions of colloidal particles. Thus 100 or more oleate ions are grouped together in a spherical way keeping their hydrocarbon parts inside and the -COO— part remains projected in water.
 Micelle Formation
Micelle Formation
(iii)Based on Physical state of Dispersed Phase & Dispersion Medium
Depending upon whether the dispersed phase and the dispersion medium are solids, liquids, or gaseous, eight types of colloidal systems are possible. A gas mixed with another gas forms a homogeneous mixture and not a colloidal system. Typical examples of various types along with their characteristic names are given in the table.
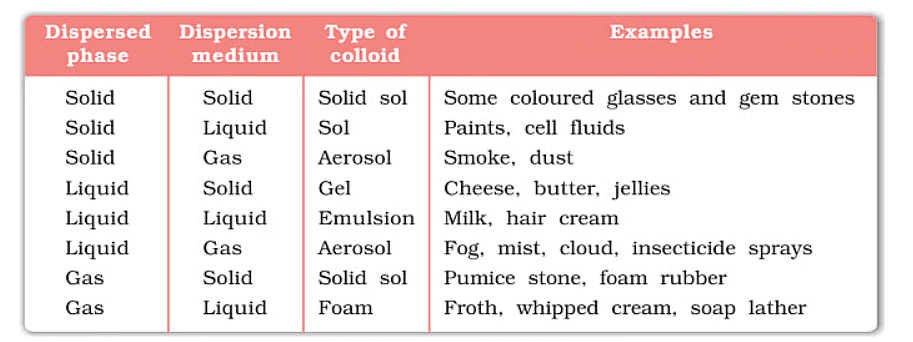 Types of Colloidal Solution
Types of Colloidal Solution
Based on Dispersion Medium
1. Water : Hydrosols
2. Alcohol : Alcohols
3. Gases : Aerosols
4. Benzene : Benzosol
5. Solid : Gel
Some colloids, such as gelatin, can behave both as a sol and a gel. At high temperatures and low concentrations of gelatin, the colloid is a hydrosol. But at low temperature and high gelatin concentration, the hydrosol can change into a gel.
|
366 videos|824 docs|301 tests
|
FAQs on Colloids & Its Classification - Chemistry for JEE Main & Advanced
| 1. What is a colloid? |  |
| 2. How are colloids classified based on the interaction between dispersed phase and dispersion medium? |  |
| 3. What determines the type of particles of the dispersed phase in colloids? |  |
| 4. Can you provide an example of a lyophilic colloid? |  |
| 5. How are colloids important in various industries and applications? |  |















