Surface Chemistry and Adsorption | Chemistry for JEE Main & Advanced PDF Download
What is Surface Chemistry?
It is the study of the chemical phenomena that occur at the interface of two surfaces which can be solid-liquid, solid-gas, solid-vacuum, liquid-gas, etc. Some applications of surface chemistry are known as surface engineering.
There are various phenomena taking place on the surface of substances and some of them are:
- Adsorption
- Heterogeneous Catalysis
- Corrosion
- Crystallization
Applications of Surface Chemistry
In a wider perspective, surface chemistry deals with the interaction of surfaces of one system with that of the other system. Some phenomena work on this principle such as:
- Catalysis
- Colloid Formation
- Electrode Reactions
- Chromatography
Surface Chemistry has a major role in various chemical processes such as:
- Enzymatic reactions at the biological interfaces found in the cell walls and membranes.
- In the electronics industry, they are used in the surface and interface of microchips found in computers.
- In automobile exhausts, the heterogeneous catalysts found in the catalytic converter for cleaning emissions.
Role of Adsorption in Surface Chemistry
Accumulation of species on higher concentration on the surface of a substance due to intermolecular force is known as adsorption. For Example, gases such as H2, O2, N2 adsorbs on the surface of activated charcoal.
Enthalpy of Adsorption: The amount of heat energy liberated when one mole of gas is adsorbed on the unit surface area of adsorbent is known as enthalpy of adsorption.
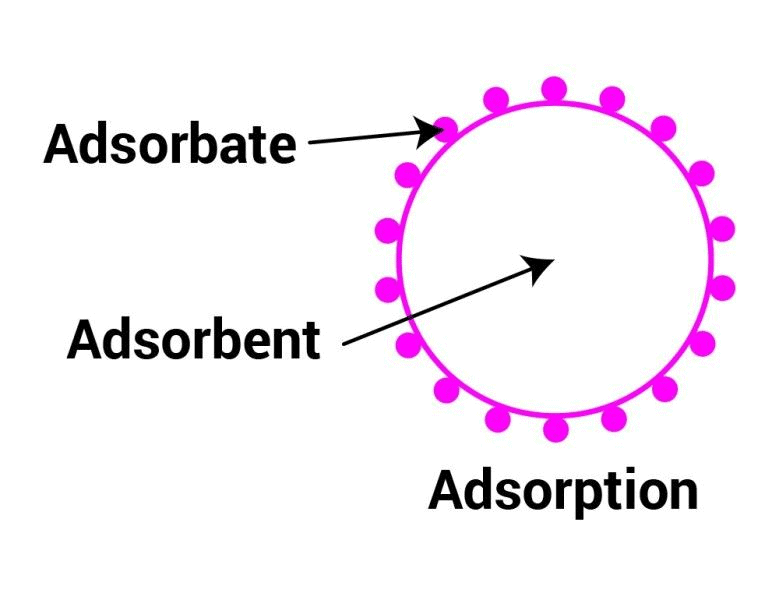
Adsorption
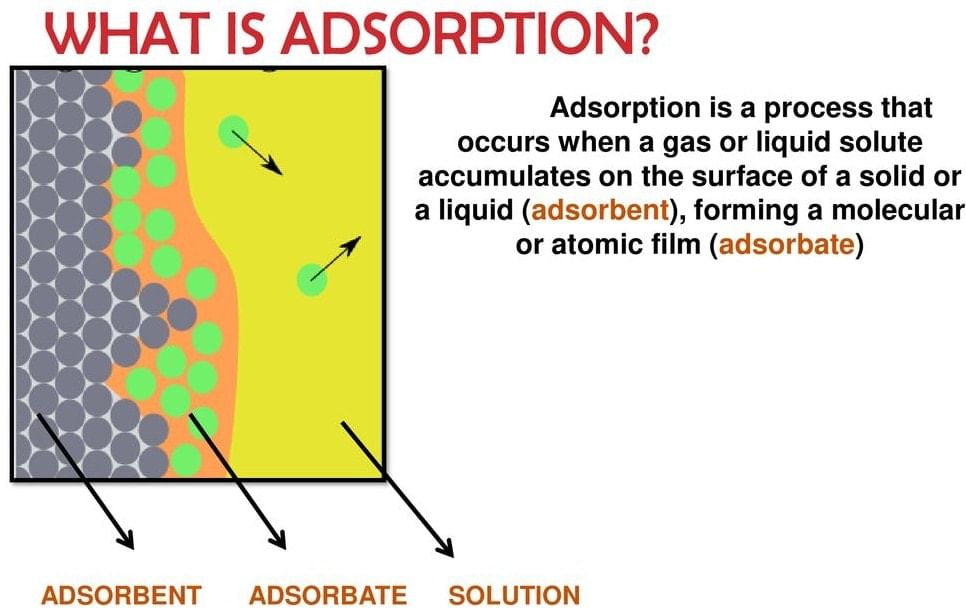
- There are several examples that reveal the fact that the surface of a solid (or liquid) has a tendency to attract and retain the molecules of another immiscible phase with which it is brought into contact. These molecules remain only at the surface and do not go deeper into the bulk.
- This tendency of accumulation of molecular species at the surface than in the bulk of a solid (or liquid) is termed adsorption. The molecular species or substance which concentrates or accumulates at the surface is termed adsorbate and the material on whose surface the adsorption has taken place is called adsorbent.
- The reverse process i.e. removal of an adsorbed substance from the surface is called desorption.
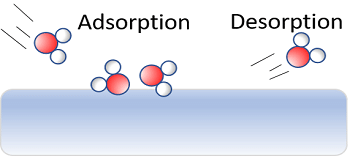
- The adsorption of gases on the surface of metals is called occlusion.
- The term sorption is employed when adsorption, as well as absorption, take place simultaneously.
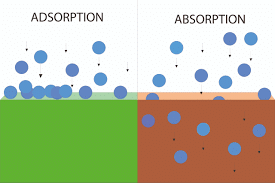
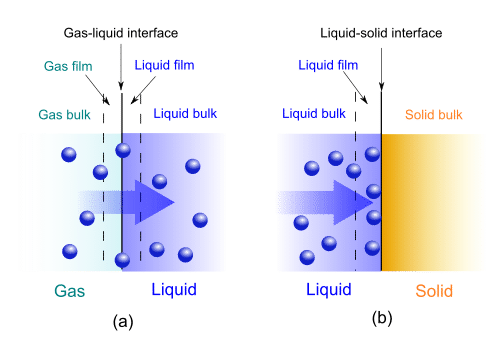 Gas-liquid absorption (a) and liquid-solid adsorption (b) mechanism. Blue spheres are solute molecules.
Gas-liquid absorption (a) and liquid-solid adsorption (b) mechanism. Blue spheres are solute molecules.
Adsorption is a process which involves the accumulation of a substance in molecular species in higher concentration on the surface. If we look at Hydrogen, Nitrogen and Oxygen, these gases adsorb on activated charcoal. Meanwhile, we have to note that adsorption is different from absorption. The two processes involve totally different mechanisms.
For the adsorption process, two components are required,
1. Adsorbate: Substance that is deposited on the surface of another substance. For example, H2, N2 and O2 gases.
2. Adsorbent: Surface of a substance on which adsorbate adsorbs. For example, Charcoal, Silica gel, Alumina.
Distinction Between Adsorption And Absorption
- In adsorption, the concentration of the adsorbate increases only at the surface of the adsorbent, while in absorption the concentration is uniform throughout the bulk of the solid
- Adsorption is due to the fact that the surface particles of the adsorbent are in a different state than the particles inside the bulk. Inside the adsorbent, all the force acting between the particles are mutually balanced but on the surface, the particles are not surrounded by atoms or molecules of their kind on all sides and hence they possess unbalanced or residual attractive forces.
- These forces of the adsorbent are responsible for attracting the adsorbate particle on its surface.
- Adsorption is a surface phenomenon, whereas absorption is a bulk phenomenon.
- Adsorption occurs only at the surface of the adsorbent, whereas absorption occurs throughout the body of the material.
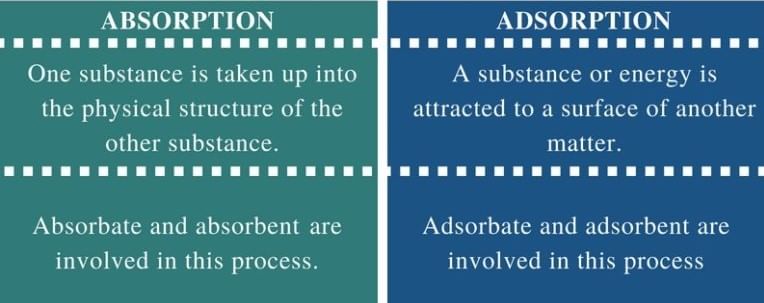 Difference between Adsorption and Absorption
Difference between Adsorption and Absorption
Mechanism of Adsorption
It is an exothermic process which means that energy is liberated during this process. The amount of heat that gets evolved when one mole of the adsorbate is adsorbed on the adsorbent is known as enthalpy. The change in enthalpy is denoted to be negative. The reason behind this is that when adsorbate molecules are adsorbed on the surface, the freedom of movement of molecules become restricted and this results in a decrease in entropy. At constant temperature and pressure, adsorption occurs spontaneously.
Types of Adsorption
Due to the force of interaction between adsorbate and adsorbent, adsorption in surface chemistry is classified into two types.
(A) Physisorption
- Nature of Forces: In physisorption, weak van der Waals forces hold the adsorbate to the adsorbent.
- Specificity: This process is not specific in nature, meaning it can occur with various substances.
- Reversibility: Physisorption is reversible, allowing the adsorbate to be easily removed from the adsorbent.
- Layer Formation: It can form multiple layers of adsorbed molecules, with each layer held by weak forces.
- Enthalpy of Adsorption: The enthalpy of adsorption is low, typically between 20 and 40 KJ/mole, indicating that little energy is released when adsorption occurs.
- Energy of Activation: The energy of activation for physisorption is very low, making the process occur readily without needing significant energy input.
- Desorption: Desorption, or the removal of the adsorbate, is very easy due to the weak forces involved.
- Factors Affecting Physisorption: Factors that affect physisorption include the surface area of the adsorbent, the nature of the adsorbate, pressure, and temperature.
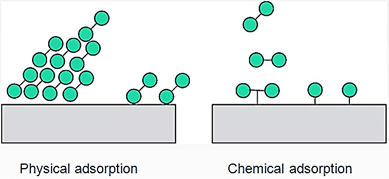 (B) Chemisorption
(B) Chemisorption
- Nature of Forces: Chemisorption is characterized by strong chemical forces between the adsorbate and the adsorbent.
- Specificity: This process is highly specific, occurring only with certain substances that can form chemical bonds with the adsorbent.
- Reversibility: Chemisorption is irreversible, meaning once the adsorbate is bound, it is difficult to remove.
- Layer Formation: Unlike physisorption, chemisorption forms a single layer of adsorbed molecules because the strong chemical forces prevent further layers from forming.
- Enthalpy of Adsorption: The enthalpy of adsorption is high, ranging from 40 to 400 KJ/mole, indicating a significant amount of energy is released when adsorption occurs.
- Energy of Activation: The energy of activation for chemisorption is also very high, meaning the process requires substantial energy to initiate.
- Desorption: Due to the strong bonds formed, desorption is very difficult.
- Factors Affecting Chemisorption: The factors influencing chemisorption include the surface area of the adsorbent, the nature of the adsorbate, and the temperature.
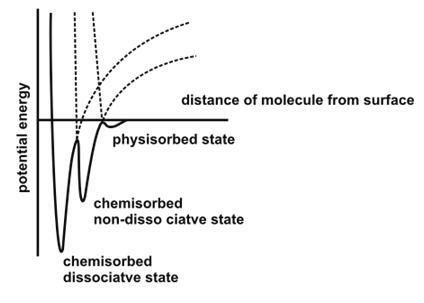 Potential energy map of Physisorption and Chemisorption
Potential energy map of Physisorption and Chemisorption
Characteristics of Adsorption
- Molecules at the surface of a solid, a metal, or a liquid experience in net inward force of attraction with free valencies.
- Adsorption is accompanied by the evolution of heat. The amount of heat evolved when one mole of a gas is adsorbed on a solid, is known as molar heat of adsorption. Its magnitude depends upon the nature of the gas.
- The magnitude of gaseous adsorption depends upon temperature, pressure, nature of the gas and the nature of the adsorbent.
- Adsorption decreases with an increase in temperature since it is accompanied by the evolution of heat.
- The adsorption increases with an increase in pressure since adsorption of gas leads to a decrease in pressure.
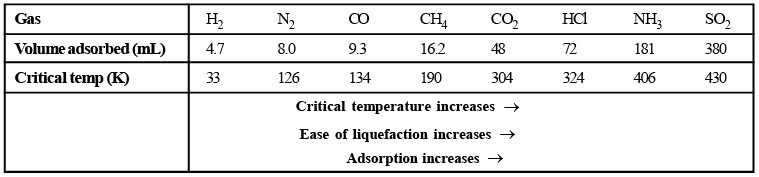
- More readily soluble and easily liquefiable gases HCl, Cl2 , SO2 and NH3 are adsorbed more than the so-called permanent gases such as H2 , O2 , N2 etc. because Vander Waal's forces involved in adsorption are much predominant in the former gases than the latter category of gases.
Factors that affect the extent of adsorption on a solid surface
Nature of the adsorbate (gas) and adsorbent (solid)
- Porous and finely powdered solid e.g. charcoal adsorb more as compared to hard non-porous materials. Due to this property powdered charcoal is used in gas masks used in coal mines.
- Gases with high critical temperature are ads or bed at higher extent as compared to gases with lower critical temperatures.
Table: Volumes of gases at N.T.P., adsorbed by 1g of charcoal at 288 K
The Surface area of the Solid Adsorbent
- The extent of adsorption depends directly upon the surface area of the adsorbent, i.e. larger the surface area of the adsorbent, the greater is the extent of adsorption.
Effect of pressure on the adsorbate gas
- An increase in the pressure of the adsorbate gas increases the extent of adsorption.
- At low temperature, the extent of adsorption increases rapidly with pressure.
- At low pressure, the extent of adsorption is found to be directly proportional to the pressure.
- At high pressure (closer to the saturation vapour pressure of the gas), the adsorption tends to achieve a limiting value.
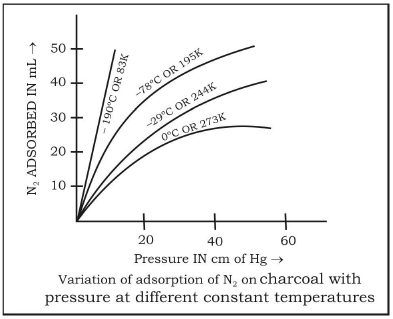
Effect of Temperature
- As adsorption is accompanied by the evolution of heat, so according to Le-Chatelier’s principle, the magnitude of adsorption should decrease with rising in temperature.
- The amount of heat when one mole of the gas is adsorbed on the adsorbent is called the heat of adsorption.
Effect of adsorption from solution
- The adsorption from solutions by solid adsorbents is found to depend upon the following factors:
(i) Nature of the adsorbate and the adsorbent.
(ii) The adsorption decreases with temperature.
(iii) Greater the surface area of the adsorbent greater is the adsorption.
(iv) Concentration of the solute in the solution.
Applications of Adsorption
The phenomenon of adsorption finds a number of applications. Important applications are given as follows:
- Production of high vacuum - A bulk of charcoal cooled in liquid air is connected to a vessel that has already been exhausted as for as possible by a vacuum pump. The remaining traces of air are adsorbed by the charcoal to produce a very high vacuum.
- In Gas masks - It is a device that consists of activated charcoal or a mixture of adsorbents. This apparatus is used to adsorb poisonous gases (e.g. Cl2, CO, oxide of sulphur etc.) and thus purify the air for breathing is coal mines.
- For desiccation or dehumidification - Certain substances have a strong tendency to absorb water such as silica and alumina (Al2O3). These substances can be used to reduce/remove water vapours or moisture present in the air. Silica gel is also used for dehumidification in electronic equipment.
- Removal of colouring matter from solution - Animal charcoal removes colours of solutions by adsorbing coloured impurities. It is also used as a decolouriser in the manufacture of cane sugar.
- Heterogeneous catalysis - Mostly heterogeneous catalytic reactions proceed through the adsorption of gaseous reactants on the solid catalyst.
Examples:
(i) Finely powdered nickel is used for the hydrogenation of oils.
(ii) Finely divided vanadium pentaoxide (V2O5) is used in the contact process for the manufacture of sulphuric acid.
(iii) Pt, Pd are used in many industrial processes as catalyst.
(iv) Manufacture of ammonia using iron as a catalyst. - Separation of inert gases - Due to the difference in the degree of adsorption of gases by charcoal, a mixture of inert gases can be separated by adsorption on coconut charcoal at different low temperatures.
- Softening of hard water - The hard water is made to pass through a column packed with zeolite (sodium aluminium silicate)
- De-ionisation of water - For softening, water can be de-ionised by removing all dissolved salts with the help of cation and anion-exchanger resin.
- In curing diseases - A number of drugs are adsorbed on the germs and kill them or these are adsorbed on the tissues and heat them.
- Cleaning agents - Soap and detergents get adsorbed on the interface and thus reduce the surface tension between dirt and cloth, subsequently, the dirt is removed from the cloth.
- Froth floatation process
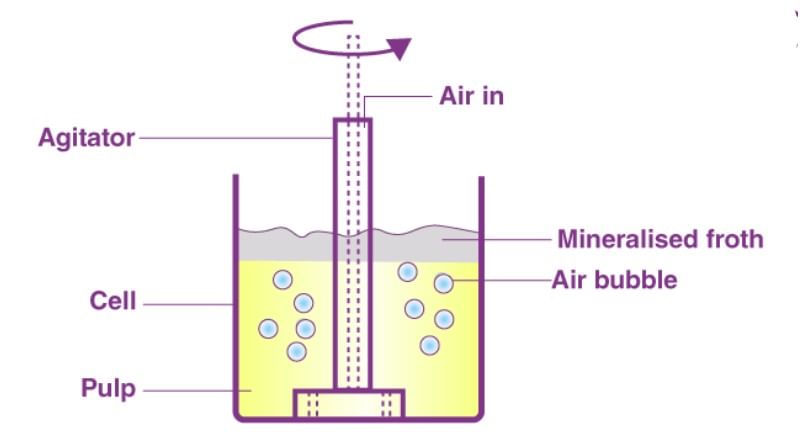 (i) A low-grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method.
(i) A low-grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method.
(ii) The finely divided ore is added to water containing pine oil and foaming agent.
(iii) The air is bubbled through the mixture.
(iv) The foam formed rises to the surface on which mineral particles wetted with oil are adsorbed while earthy matter settles down at the bottom. - In adsorption indicators - The surface of certain precipitates such as silver halide has the property of adsorbing some dyes like eosin, fluorescein etc. In this case of precipitation titrations (for example AgNO3 Versus NaCl) the indicator is adsorbed at the endpoint producing a characteristic colour on the precipitate.
- Chromatographic analysis - The phenomenon of adsorption has given an excellent technique of analysis known as chromatographic analysis. The technique finds a number of applications in analytical and industrial fields. The chromatographic technique is based on differential adsorption of different constituents of a mixture.
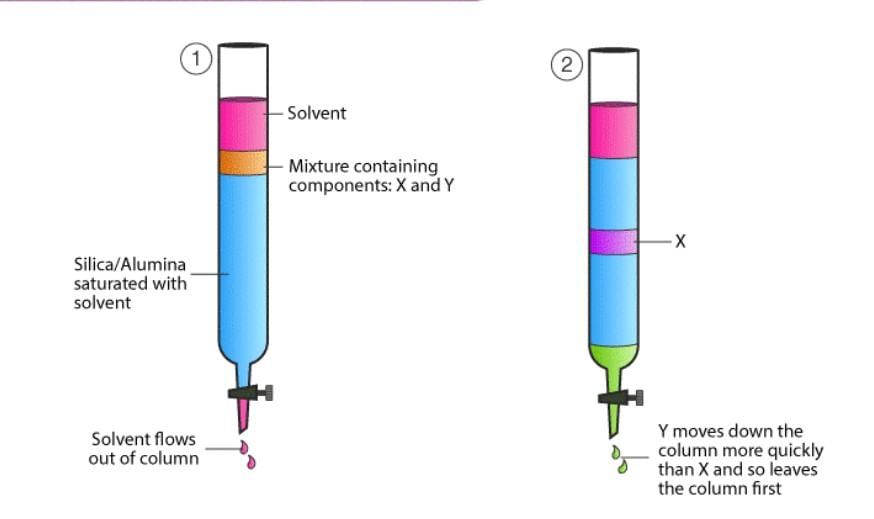 Absorption Chromatography
Absorption Chromatography
- In dyeing - Many dyes get adsorbed on the cloth either directly or by the use of mordants. “Catalyst is a substance which speeds up and speeds down a chemical reaction without itself being used up at the end of the reaction and the phenomenon is known as catalysis.
|
334 videos|660 docs|300 tests
|
FAQs on Surface Chemistry and Adsorption - Chemistry for JEE Main & Advanced
| 1. What is the significance of surface chemistry in various industries? |  |
| 2. How does adsorption contribute to the field of surface chemistry? |  |
| 3. What are the different types of adsorption that are commonly studied in surface chemistry? |  |
| 4. How do factors such as surface area and surface energy impact the extent of adsorption on a solid surface? |  |
| 5. Can temperature affect the adsorption process in surface chemistry? If so, how? |  |

















