Adsorption Isotherms, Adsorption from Solution Phase & Catalysis | Chemistry for JEE Main & Advanced PDF Download
What is Adsorption Isotherm?
The variation of adsorption with pressure at a constant temperature is called adsorption isotherm. Adsorption is usually described by isotherms. It is due to the fact that temperature plays an important role or that it has a great effect on the whole process.
Different Isotherm Models
1. Freundlich adsorption isotherm
Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship can be expressed by the following equation.
At constant temperature x/m = k · P1/n
where `x' is the mass of the gas adsorbed on a mass `m' of the adsorbent at a pressure P. k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
At low pressure, the amount of the gas adsorbed per unit quantity of adsorbent is proportional to the pressure. At high pressure, the amount of adsorbed gas is independent of pressure. At intermediate pressures, Freundlich adsorption isotherm is expected to hold
2. Langmuir Adsorption Isotherm
According to Langmuir —
(a) There is adsorption of gas molecules on the surface of the solid.
(b) There is desorption of the adsorbed molecules from the surface of the solid.
(c) There is formation of unimolecular layer and thus it is chemisorption
(e) A dynamic equilibrium is attained when rate of adsorption = rate of desorption.
(f) Based on the above facts, langmuir adsorption isotherm is represented as
Case-I At very high pressure
bp >>> 1, hence 1 + bp ≈ bp
Case-II At very low pressure
bp <<< 1
1 + bp ≈ 1
θ = ap,
θ ∝ p
This is shown along OA
3. BET theory (after Brunauer, Emmett, and Teller)
The BET theory was proposed by Brunauer, Emmett and Teller in the year 1938. This theory explains the formation of multilayer adsorption during physisorption. This theory also talks about the uniformity in the sites of adsorption of solid surfaces. It assumes that when adsorption occurs at one site it will not affect adsorption at neighbouring sites.
Adsorption from Solution Phase
Solid surfaces can also adsorb solutes from solutions.- When the litmus solution is shaken with charcoal, it becomes colourless because the dye of the litmus solution is adsorbed by charcoal.
- When the colourless Mg(OH)2 is precipitated in the presence of magneson reagent (a blue coloured dye). it acquires blue colour because the dye is adsorbed on the solid precipitate. The extent of adsorption from the solution depends upon the concentration of the solute in the solution and can be expressed by the Freundlich isotherm. The Freundlich adsorption isotherm for the adsorption from solution is, xm = kc1/n where x is the mass of the solute adsorbed, m is the mass of the solid adsorbent, c is the equilibrium concentration of the solute in the solution, n is a constant having value greater than one. k is the proportionality constant, (The value of k depends upon the nature of solid, its particle size, temperature, and the nature of solute and solvent etc.) The plot of x/m against c is similar to that of the Freundlich adsorption isotherm.
The above equations may be written in the following form: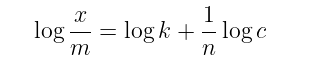 where c, is the equilibrium concentration of the solute in the solution.
where c, is the equilibrium concentration of the solute in the solution.
Catalysis
- All reactants need to overcome certain energy, better known as activation energy in order to form products.
- This activation energy is the difference between the energy of the transition state and the reactant species. Some reactant molecules have enough kinetic energy to overcome this energy barrier whereas others don’t.
- Hence, not all reactions happen at the same rate in general conditions. Therefore, certain reagents are added which lower the required activation energy for the conversion of reactants to products.
- These reagents are known as catalysts and this process of lowering the activation energy is known as catalysis.
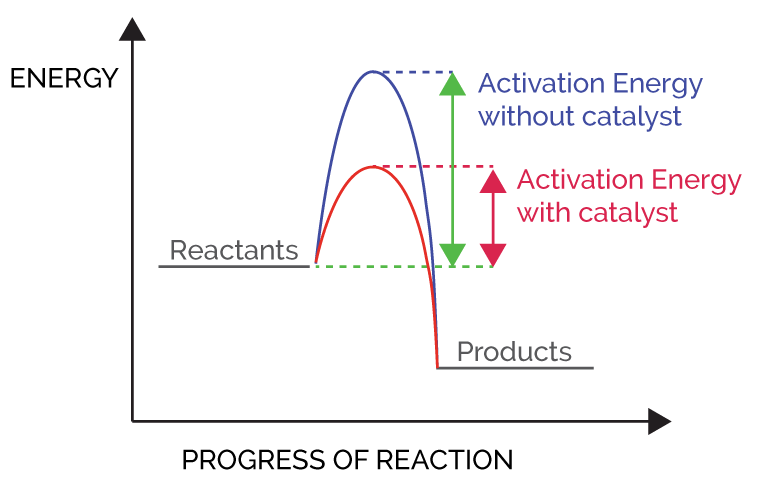
- Catalysis of chemical reactions increases the rate of chemical reaction with the help of a catalyst.
- Catalysts increase the rate of a reaction without undergoing any chemical or physical change.
- As discussed above, catalysts just decrease the energy barrier for the conversion of reactants to products.
Types of Catalysis
Catalysis of chemical reactions is generally divided into two categories:
- Homogeneous Catalysis: Homogeneous catalysis of chemical reactions is a process where the reactants involved in the reaction and the catalyst are in the same phase. For example hydrolysis of sugar in the presence of sulphuric acid.
- Heterogeneous Catalysis: Heterogeneous catalysis of chemical reactions is a process where the reactants involved in the reaction and the catalyst are in different phases. For example reaction of hydrogen and nitrogen in the presence of finely divided iron to form ammonia.
Mechanism of Heterogeneous Catalysis of Chemical Reactions:
The modern theory of adsorption proposed a five-step mechanism for the catalysis of chemical reactions. These steps are:
- Introduction and diffusion of reactant molecules on the catalytic surface.
- Adsorption of molecules of reactants on the catalytic surface.
- Formation of intermediate on a catalytic surface by a chemical reaction between the reactant molecules.
- Desorption of product molecules from the catalytic.
- Diffusion of product molecules away from the catalytic surface to form final products.
Mechanism of Enzyme Catalysis
- The mechanisms of enzyme catalysis usually vary in different processes. However, they are quite similar in principle to other types of chemical catalysis.
- There is a reduction of energy barrier(s), therefore, separating the reactants from the products. With reduced activation energy the fraction of reactant molecules that can overcome this barrier increases and the product is formed.
- An important factor that we need to consider is that enzymes can catalyze reactions in both directions but cannot take a reaction forward nor change the equilibrium position. The enzyme is not changed or consumed during the reaction and it can perform many catalyses repeatedly.
- Meanwhile, the most common mechanism that explains the activity of enzymatic catalysis is the lock and key mechanism.
Important concepts of lock and key mechanism:
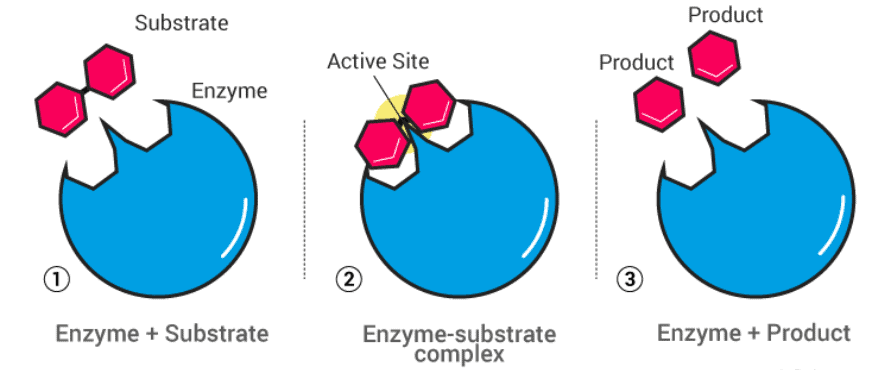
- This mechanism exactly explains the action of an enzyme.
- The enzyme surface exactly like a key.
- Its surface has so many active centres with many fructose groups.
- Substrate molecule has a shape exactly like a lock, this enzyme fits into the substrate molecule where the exchange of functional groups takes place.
- This leads to the formation of an enzyme-substrate complex which transforms into an enzyme product complex.
- This enzyme product complex dissociates into enzyme and products.
FAQs:
1. Adsorption of gas on a solid is always exothermic explain?
Adsorption of gas on a solid is a spontaneous process. When a gas adsorbs on the solid, due to molecular interaction process, the entropy of gas molecules decreases. To make the process spontaneous, adsorption must be exothermic.
2. Among SO2, CH4, H2 gases which gas adsorbs more on the charcoal and why?
Sulphur dioxide adsorbs more than methane and hydrogen since the critical temperature of sulphur dioxide is higher than methane and hydrogen.
3. What do you mean by critical temperature? What is the relation of critical temperature and the gas adsorbed?
The temperature above which a gas cannot be liquefied even by the application of high pressure is known as critical temperature. Greater is the critical temperature (To) more in the gas adsorbed on the surface.
4. What are the factors of effective adsorption of gases on solids?
Nature of gases: Ease is liquefication more is gas adsorbed.
The surface area of adsorbent: More the surface area more gas is adsorbed.
Temperature: Physisorption increases with a decrease in temperature while chemisorption increases with an increase in temperature.
Pressure: It affects only physisorption if pressure increase amount of gas adsorbs also increase.
5. Define activation.
When adsorbents are heated in a vacuum from 573 – 623 K, the surface area is increased and it is known as activation.
6. Write about adsorption from solutions?
Charcoal pieces are added to the acetic acid solution. Some of the acetic acid molecules adsorb on charcoal. This depends upon,
- Nature of adsorbate
- The surface area of the adsorbent
- Temperature
- Concentration
|
334 videos|660 docs|300 tests
|
FAQs on Adsorption Isotherms, Adsorption from Solution Phase & Catalysis - Chemistry for JEE Main & Advanced
| 1. What is an adsorption isotherm? |  |
| 2. What is the Freundlich adsorption isotherm? |  |
| 3. What is the Langmuir adsorption isotherm? |  |
| 4. What is the BET theory? |  |
| 5. What is the mechanism of heterogeneous catalysis? |  |

















