Covalent Bond Fission: Homolytic & Heterolytic | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Homolytic Bond Fission: Homolysis |

|
| Hetrolytic Bond fission |

|
| Intermediates of organic compounds |

|
| Carbocation |

|
| Carbanion |

|
| Carbon Free Radical |

|
Homolytic Bond Fission: Homolysis
The bond cleavage in which each bonded atom gets their own contribution.
- Cleavage takes place due to:
HELP (H = Heat, E = Electricity, L = light, P = Peroxide) - Favoured when E.N. difference is less or zero.
- Cleavage favoured in non polar solvent.
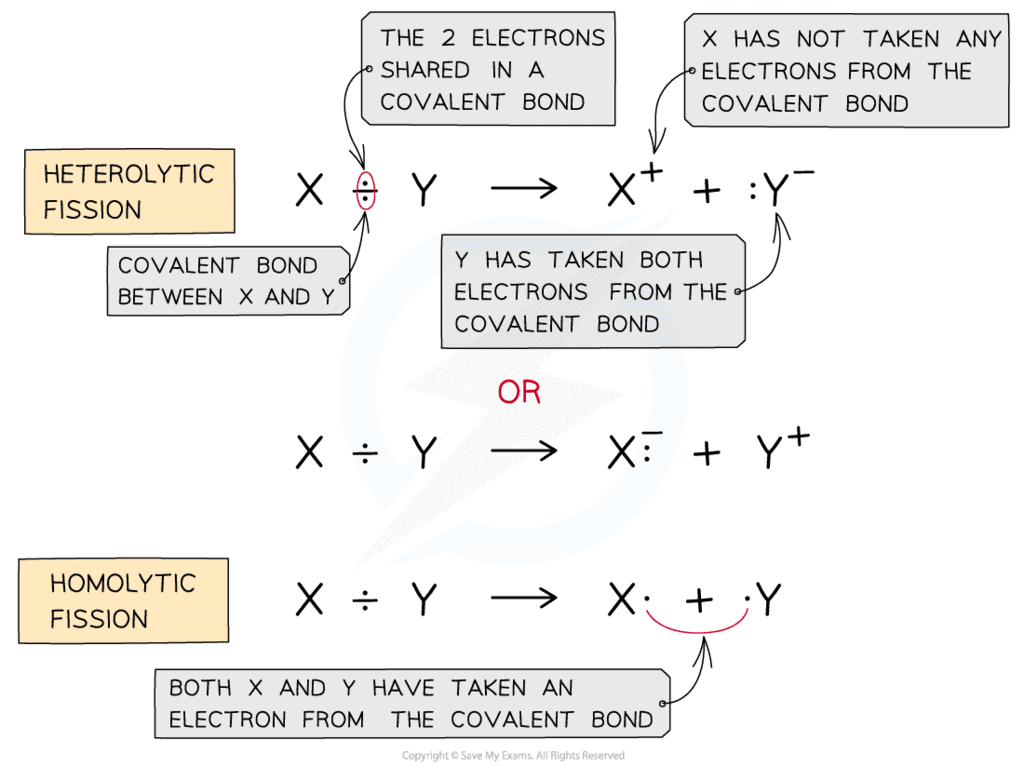
Hetrolytic Bond fission
- It is formed when the electronegativity difference between the bonded atoms is more.
- the formation is favoured by polar solvent.
+ve charge of the solvent attracts the -ve pole of the compound and the -ve pole of the solvent attracts +ve pole of the compound and the bond breaks.
Intermediates of organic compounds
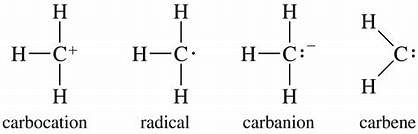
Carbocation
Carbocation
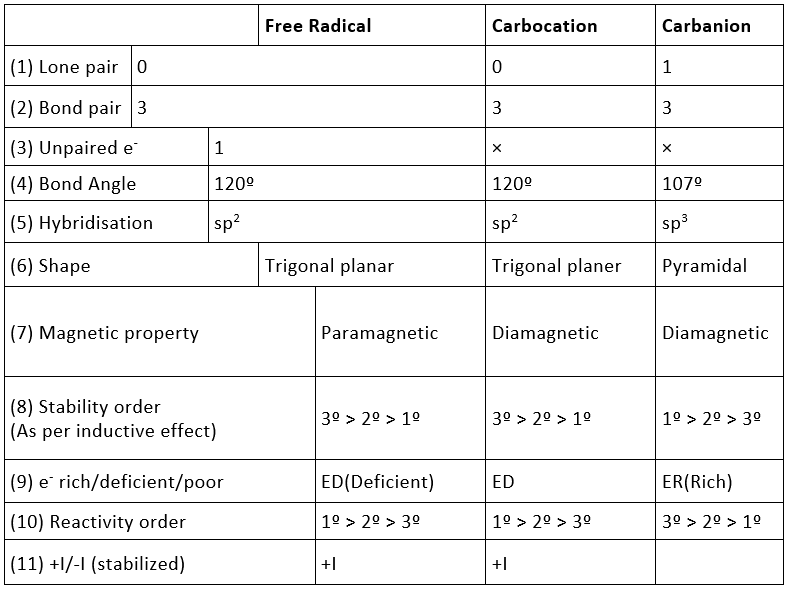
Carbocation is the intermediate of carbon containing positive charge. It has six electrons in the valence shell.
Properties of Carbocation :
1. It is positively charged species.
2. It has sixtet of electrons i.e. diamagnetic.
3. It is formed by heterolysis.
4. It is generally formed due to polar solvent.
Geometry and Hybridization
Hybridization of C⊕ = sp2
Geometry of C⊕ = Trigonal Planar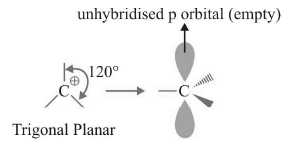
Classification of Carbocations
This classification will also be used for carbanions and carbon free radical and will be studied only in this section.

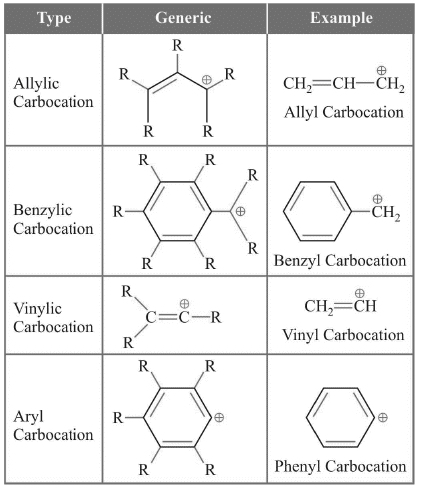 Stability of Carbocations
Stability of Carbocations
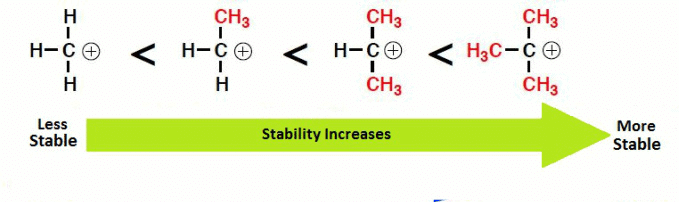
There are three factors contributing to the stability of carbocations:
(a) Inductive Effect
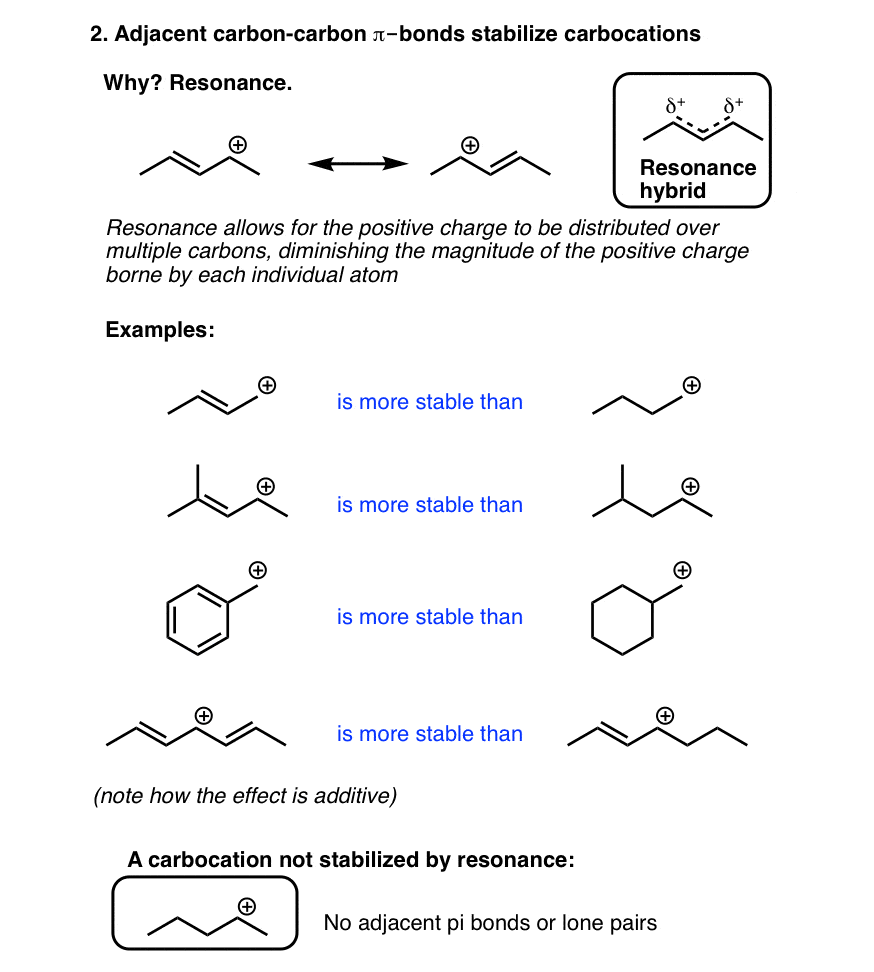
(b) Hyperconjugation
(c) Resonance
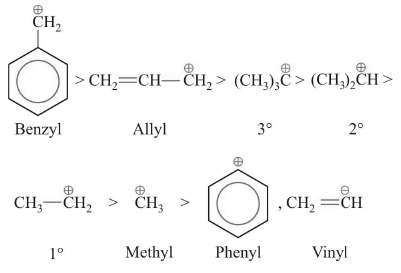
 Order of stability:
Order of stability:
Q.1. Compare the stability of the following carbocation:
(a) (b)
(c)
→ more s character
→ more electronegativity
→ +ve charge on more electronegative element is symbol of unstability.
a > b > c
Q.2. Compare the stability of the following compounds:
(a) (b)
(c)
(d)
Sol. d > c > b > a
Being most electron attracting group it decreases the e- density from positively charged C-atom and decreases the charge density and makes the carbocation less stable.
Q.3. Compare the stability of the following carbocation :
(a) (b)
(c)
(d)
Sol. Due to greater size of Iodine, its L.P. will not be available for coordinate bond. Therefore L.P. would not stabilize carbocation.
In case of F due to its small size its lone pair can be easily coordinated to making it most stable.
a > b > c > d (Stability)
* By coordination the carbocation completes its octet and structure having complete octet of its atom is supposed to be most stable.
(Each atom has its full octet)
*
Note : In Resonating Structure of at least one C gets sixtet of e-s and hence less stable than coordinated compound.
Q.4. Compare the stabilities of the following carbocation:
(a) (b)
(c)
Sol. N, O, F belongs to same period
→ In period Electronegativity of the atom is deciding factor
→ F being most electronegative, holds its e- pair very firmly.
→ Its L.P. will not be easily available for coordination.
→ Stability by it will be minimum.
a > b > c
Q.5. Compare the following carbocation in order of their stability.
(a) (b)
Sol. If periods of atoms which have to donate their electrons for coordination (for stability) is different then atomic size will be deciding factor. The atom whose size is greater will be unable to make its e- pair available for coordination.
b > a.
Q.6. Compare the stability of the following compounds:
(a) (b)
(c)
Sol → more s-character
→ more e.n.
→ attracts e-
→ reduces stability
b > a > c
Formation of Carbocations
Ionization of Carbon-Leaving Group Bond
In this method
(a) Bond between carbon and leaving group ionizes.
(b) Leaving group accepts the pair of electrons that were shared in the covalent bond.
Rate of formation of carbocation depends on
(a) The stability of carbocation formed.
(b) The nature of the leaving group. Weaker the base better the leaving group. This is because weaker leaving group implies a stable compound and its formation will therefore be favoured.
Addition of Proton to a π bond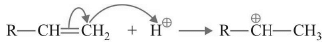
Rate of carbocation formation depends on
(a) Stability of carbocation formed.
(b) Strength of the electrophile.
Reactions of Carbocations
There are three important reactions of carbocations:
(a) Capture a Nucleophile
(b) Lose a proton to form a π bond.
(c) Rearrangement
Capture a Nucleophile

Loose a proton to form a π bond
Ex. Form the products from the following reaction: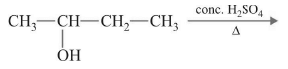
Sol.
Step-1 : Protonation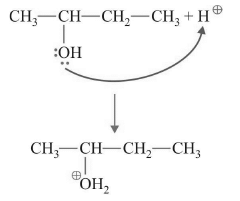
Step-2 : Formation of Carbocation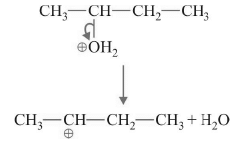
Step-3 : Deprotonation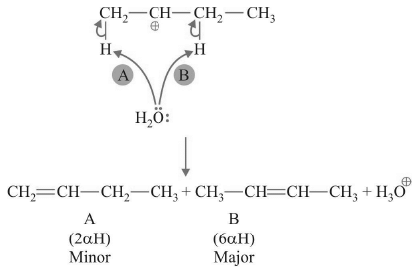
When carbocation deprotonation can lead to more than one product, all products are formed and the most stable product is the major product.
Carbocation Rearrangement
A carbocation can become more stable by rearrangement. Bonding electrons of carbocation may shift between adjacent atoms to form more stable carbocation. There are two kinds of shifts that take place in order to gain stability.
(a) Hydride Shift (b) Alkyl Shift
Carbanion
Carbanion is the intermediate of carbon containing negative charge. It has eight electrons in the valence shell.
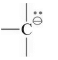
Geometry and Hybridization
Hybridization of 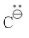 sp3
sp3
Geometry : Trigonal Pyramidal
Carbanion and ammonia are isoelectronic species having same structure
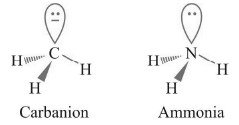
Stability
ERG will increase the electron density at carbon and will make it unstable. EWG will decrease the electro density at carbon and will make it stable.
Order of Stability
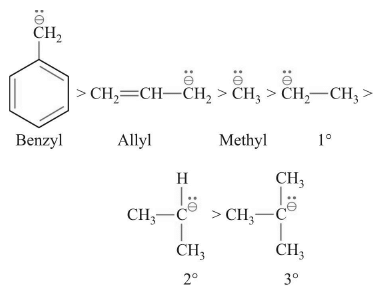
Formation of Carbanion
There are two methods for the formation of carbanion:
(A) Partial formation via Grignard Reagent
(B) Formation from Carbonyl Compounds
We will discuss these methods in the subsequent section
Formation via Grignard Reagent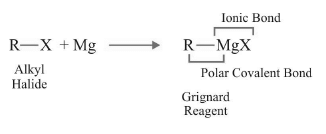

(i) Carbanion is never formed as an intermediate that can be isolated in the case of Grignard Reagent. It directly participates in the reaction.
(ii) Dry ether is used in this formation as it is inert to Grignard reagent. For the formation of Grignard reagent from aryl halides, we use tetrahedrofuran (THF) as solvent.

Formation from Carbonyl Compounds
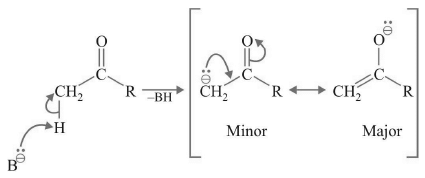
There are three reasons for the easy formation of carbanion from carbonyl compounds
(a) Resonance - stabili zation of carbanion which is the conjugate base of carbonyl compound.
(b) Hyperconjugation makes the αC-H bond acidic.
(c) –I of increases the acidic strength of C–H bond.
Reactions of Carbanion
The reactions of carbanion are very fast as electropositive carbon carries negative charge.
Grignard Reagent as a Base
In this reaction it captures acidic hydrogen.
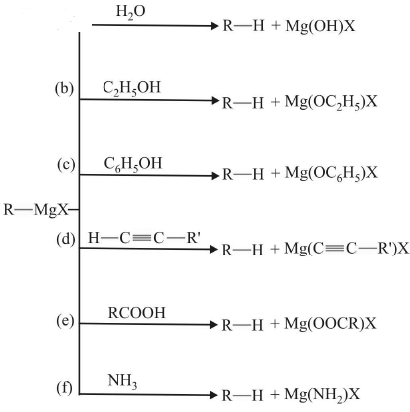
In (d), the reaction with terminal alkyne also takes place as sp-hydridized carbon is highly electronegative and therefore H attached to it is fairly acidic.
Grignard Reagent as a Nucleophile
Grignard Reagent reacts with carbonyl compounds to form alcohols. This is a very important method for the synthesis of alcohols.
Mechanism: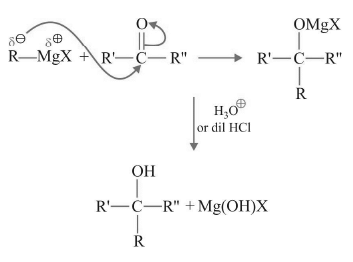
H3O⊕is never added along with the first step as Grignard Reagent will react with H⊕ to give as in previous reaction.
Aldol Condensation
This reaction is shown by carbonyl compounds containing atleast one αH in presence of dilute base such as dilute NaOH.
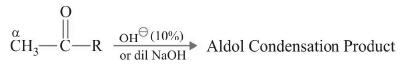
Mechanism: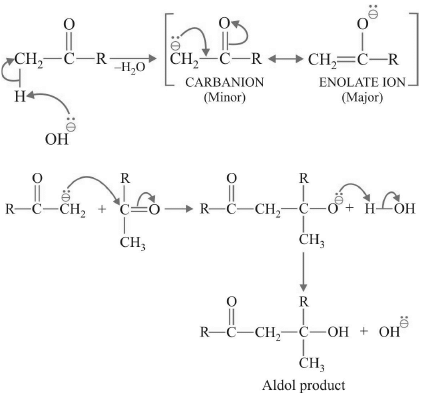
Q.1.
a > b (stability)
* Stability of the carbanion is as follows
Q.2. Compare the stability of the following carbocation:
(a) (b)
(c)
Sol. c > a > b
Q.3. Compare the stability of the following carbanion:
(a)
(b)
(c)
→ become more stable
Sol. b > a > c
Q.4. Compare the stability of the following carbanion:
(a) (b)
(c)
Sol. a > b > c
Q.5. Arrange the following anion in order of their stability:
(a) Cl-, (b) Br- (c) F- (d) I- (maximum size)
→ maximum dispersion of -ve charge
→ max stability
Sol. d > b > a > c
Q.6. Compare the stability of the following:
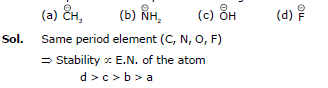
Q.7. Compare the acidic strength:
(a) HCl (b) HF (C) HBr (D) HI
Sol. Acidic strength ≈ stability of the anion formed (conjugate base)
As we know, I- > Br- > Cl- > F-
→ HI > HBr > HCl > HF
Q.8. Compare the Acidic strength of the following:
(a) NH3 (b) pH3 (c) AsH3 (d) SbH3 (e) BiH3
Sol. Anion formed from these acids are:
→ acidic strength e > d > c > b > a
Q.9. Compare the acidic strength of the following compounds:
CH4, NH3, H2O, HF
Sol. The conjugate base of the given acid is as follows:
we have already proved that
>
>
>
(Stability)
→ HF > H2O > NH3 > CH4 (acidic strength)
Q.10. Compare the stability of the following carbanion.
(a)
(b)
(c)
(d)
Sol. d > c > b > a
* M or -M is not distance dependent
Q.11. compare the stability of the following carbocation
(a)
(b)
(c)
(d)
Sol. a > b > c > d
Q.12. Compare the stability of the following carbocation.
(a)
(b)
(c)
(d)
Sol. M (OH) > M (OCH3)
b > c > d > a
Q.13. Compare the stability of the following carbocation
(a)
(b)
(c)
Sol. c > a > b
Q.14. Compare order of dehydration of the following alcohols :
(a)
(b)
(c) C - C - C - OH
Sol. After formation of carbocation
,
Since 3° carbocation is most stable therefore it will show greatest tendency to lose water as after loss of water it comes in stable form.
Carbon Free Radical
Carbon Free Radical is the intermediate of carbon having an odd electron. It is neutral and has seven electrons in the valence shell. It is highly reactive as it requires only one electron to complete its octet and therefore, is short-lived.

Geometry and Hybridization
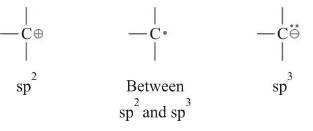 The hybridization of carbon free radical was proposed after experimental verification of structure of different radicals.
The hybridization of carbon free radical was proposed after experimental verification of structure of different radicals.
It was proposed that when ERG are placed on C•, it has sp2 hybridization and when EWG are placed on C•, it has sp3 hybridization.

Stability
ERG increase stability while EWG decrease stability.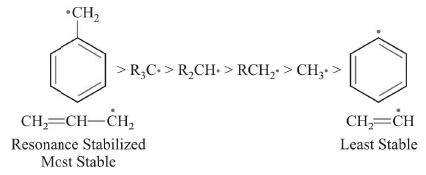
Formation of Carbon Free Radical
Carbon Free Radicals are formed by homolytic cleavage of bonds. They are formed:
(a) at high temperature in the gas phase
(b) in non-polar solvents
(c) by ultraviolet light
(d) by addition of other radicals
Reactions of Carbon Free Radical
The most common reactions in which free radical is involved are:
(a) Halogenation of alkanes.
(b) Addition of HBr in the presence of peroxides to alkenes. (Anti-Markonikov Rule)
(c) α-Halogenation of alkenes.
(d) Wurtz Reaction
(e) Decarboxylation reaction
Free Radical Halogenation of Alkanes
The typical reactions of free radical are chain reaction mechanisms. There are three steps in a chain reaction mechanism: initiation, propagation and termination.
Mechanism
(I) Chain Initiation
There are two choices:
(a) 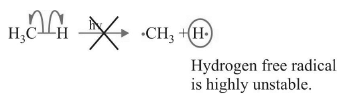
(b) 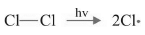
(I) (b) will take place as it is energetically feasible.
(II) Chain Propagation
(a)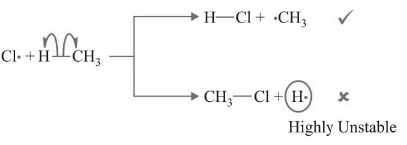
(b)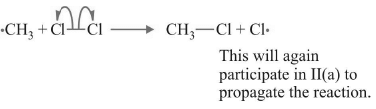
(III) Chain Termination
The various free radicals in the progress of reaction are:
Cl•, CH3•. There are ways in which free radicals from different chains may combine to terminate the reaction.
CH3• + CI → CH3 - CI
CH3• + CH3• → CH3 - CH3
CI• + CI• → CI2
There is a side-product CH3 - CH3 that is formed in this reaction. Besides these, a number of other side-products such as CH2Cl2, CHCl3 and CCl4 have also been observed. These get formed since carbon free radical is highly reactive. It randomly reacts with other species that make it stabler.
|
334 videos|651 docs|300 tests
|
FAQs on Covalent Bond Fission: Homolytic & Heterolytic - Chemistry for JEE Main & Advanced
| 1. What is homolytic bond fission? |  |
| 2. How does heterolytic bond fission differ from homolytic bond fission? |  |
| 3. What are intermediates of organic compounds? |  |
| 4. What is a carbocation? |  |
| 5. What is a carbanion? |  |





















