Carbocation & Carbanions | Chemistry for JEE Main & Advanced PDF Download
Carbocation
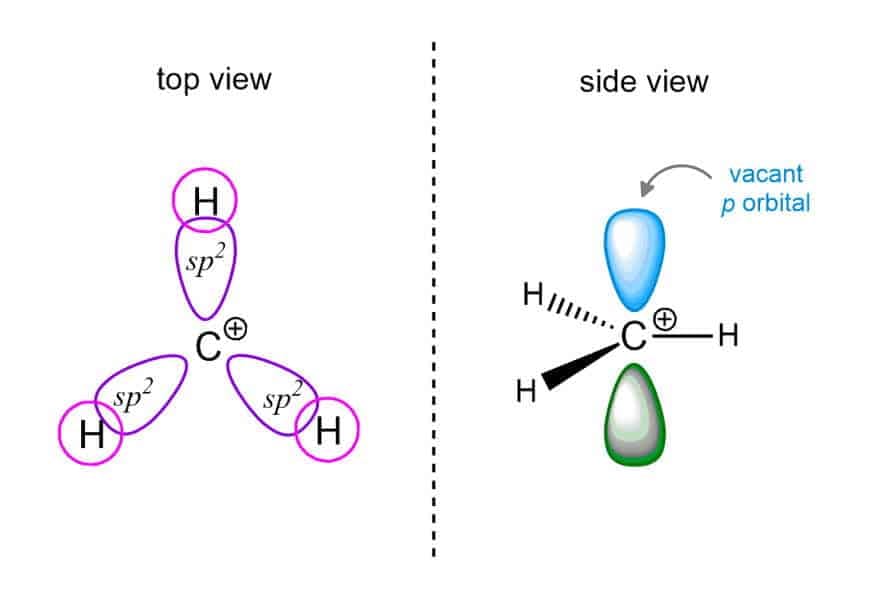
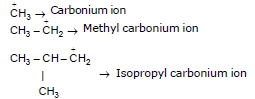
Properties of Carbocation :
1. It is positively charged species.
2. It has a sextet of electrons i.e. diamagnetic.
3. It is formed by heterolysis.
4. It is generally formed due to polar solvent.
Structure :
(sp2), Triangular planar
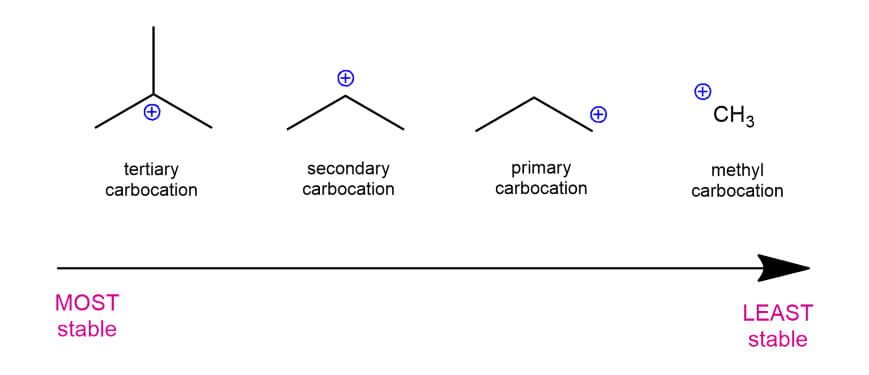
Stability :
Its stability can be determined with the help of Inductive effect, Hyperconjugation and Resonance effect.
Stability of Carbocation:
The stability of carbocation can also be determined by Hyperconjugation (no bond Resonance).
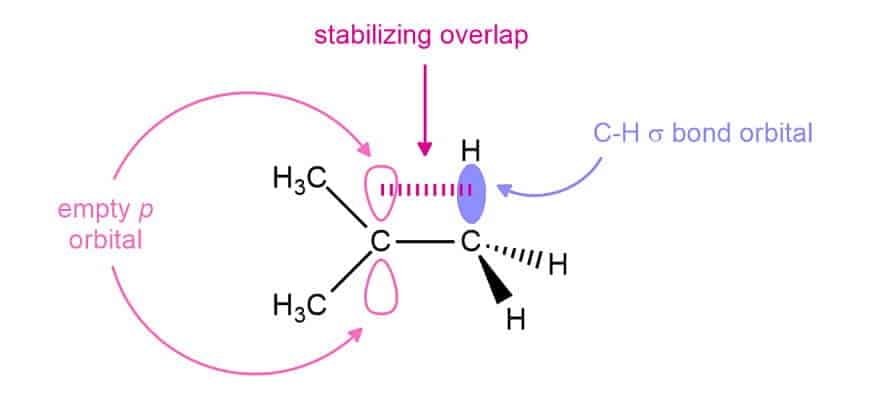
9 α C - H bond 6αC - H bond 3α C - H bond
Allylic Carbocation:
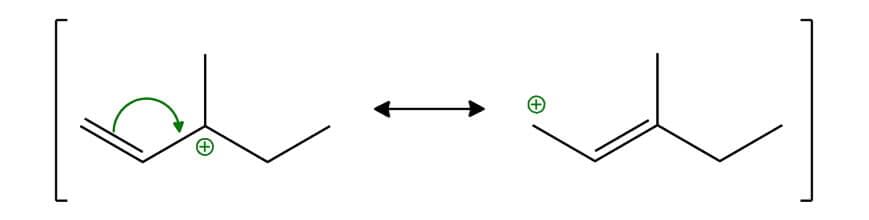
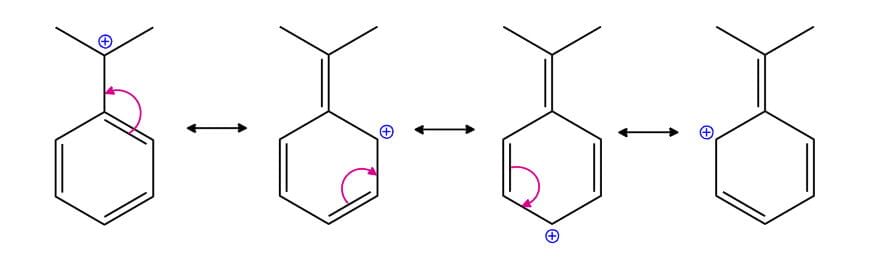
Benzylic Carbocation:
Q.1. Compare the stability of the following carbocation:
(a) (b)
(c)
→ more s character
→ more electronegativity
→ +ve charge on more electronegative element is symbol of unstability.
a > b > c
Q.2. Compare the stability of the following compounds:
(a) (b)
(c)
(d)
Sol. d > c > b > a
Being most electron attracting group it decreases the e- density from positively charged C-atom and decreases the charge density and makes the carbocation less stable.
Q.3. Compare the stability of the following carbocation :
(a) (b)
(c)
(d)
Sol. Due to greater size of Iodine, its L.P. will not be available for coordinate bond. Therefore L.P. would not stabilize carbocation.
In case of F due to its small size its lone pair can be easily coordinated to making it most stable.
a > b > c > d (Stability)
* By coordination the carbocation completes its octet and structure having complete octet of its atom is supposed to be most stable.
(Each atom has its full octet)
*
Note : In Resonating Structure of at least one C gets sixtet of e-s and hence less stable than coordinated compound.
Q.4. Compare the stabilities of the following carbocation:
(a) (b)
(c)
Sol. N, O, F belongs to same period
→ In period Electronegativity of the atom is deciding factor
→ F being most electronegative, holds its e- pair very firmly.
→ Its L.P. will not be easily available for coordination.
→ Stability by it will be minimum.
a > b > c
Q.5. Compare the following carbocation in order of their stability.
(a) (b)
Sol. If periods of atoms which have to donate their electrons for coordination (for stability) is different then atomic size will be deciding factor. The atom whose size is greater will be unable to make its e- pair available for coordination.
b > a.
Q.6. Compare the stability of the following compounds:
(a) (b)
(c)
Sol → more s-character
→ more e.n.
→ attracts e-
→ reduces stability
b > a > c
Carbanion
1. It is a -ve charged species 2. It has octet of electrons. 3. diamagnetic
Structure :
* If -ve charge is in Resonance then the hybridisation of carbanion is sp2(Triangular planar shape).
* If -ve charge is not in Resonance then the hybridisation of carbanion is sp3(pyramidal).
Stability :
Its stability can be determined with the help of
(1) Inductive effect
(2) Resonance effect
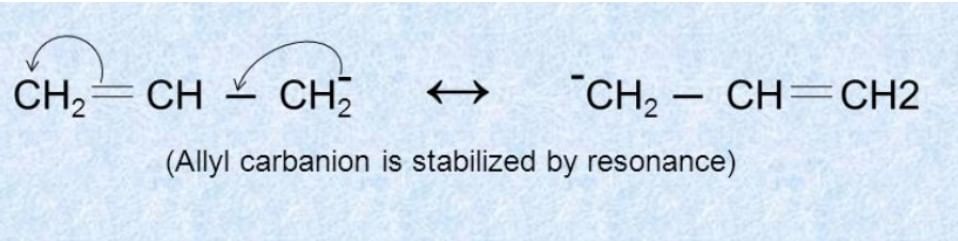
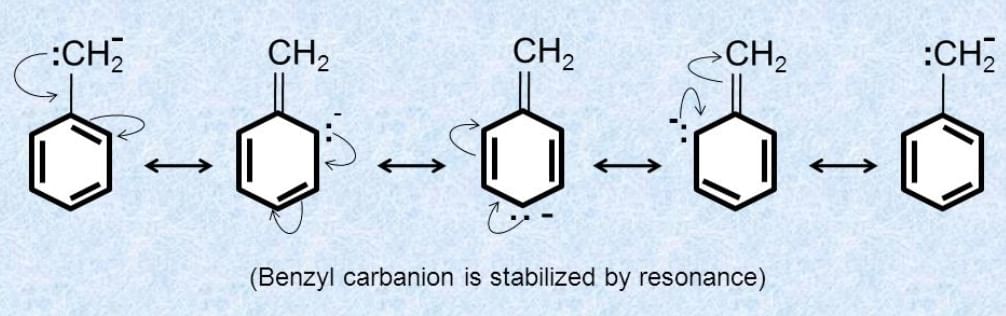
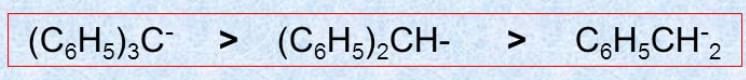
Q.1.
a > b (stability)
* Stability of the carbanion is as follows
Q.2. Compare the stability of the following carbocation:
(a) (b)
(c)
Sol. c > a > b
Q.3. Compare the stability of the following carbanion:
(a)
(b)
(c)
→ become more stable
Sol. b > a > c
Q.4. Compare the stability of the following carbanion:
(a) (b)
(c)
Sol. a > b > c
Q.5. Arrange the following anion in order of their stability:
(a) Cl-, (b) Br- (c) F- (d) I- (maximum size)
→ maximum dispersion of -ve charge
→ max stability
Sol. d > b > a > c
Q.6. Compare the stability of the following:
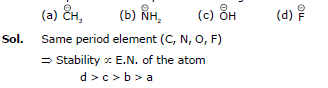
Q.7. Compare the acidic strength:
(a) HCl (b) HF (C) HBr (D) HI
Sol. Acidic strength ≈ stability of the anion formed (conjugate base)
As we know, I- > Br- > Cl- > F-
→ HI > HBr > HCl > HF
Q.8. Compare the Acidic strength of the following:
(a) NH3 (b) pH3 (c) AsH3 (d) SbH3 (e) BiH3
Sol. Anion formed from these acids are:
→ acidic strength e > d > c > b > a
Q.9. Compare the acidic strength of the following compounds:
CH4, NH3, H2O, HF
Sol. The conjugate base of the given acid is as follows:
we have already proved that
>
>
>
(Stability)
→ HF > H2O > NH3 > CH4 (acidic strength)
Q.10. Compare the stability of the following carbanion.
(a)
(b)
(c)
(d)
Sol. d > c > b > a
* M or -M is not distance dependent
Q.11. Compare the stability of the following carbocation
(a)
(b)
(c)
(d)
Sol. a > b > c > d
Q.12. Compare the stability of the following carbocation.
(a)
(b)
(c)
(d)
Sol. M (OH) > M (OCH3)
b > c > d > a
Q.13. Compare the stability of the following carbocation
(a)
(b)
(c)
Sol. c > a > b
Q.14. Compare the order of dehydration of the following alcohols :
(a)
(b)
(c) C - C - C - OH
Sol. After the formation of carbocation
,
Since 3° carbocation is most stable therefore it will show the greatest tendency to lose water as after the loss of water it comes in a stable form.
|
366 videos|824 docs|301 tests
|
FAQs on Carbocation & Carbanions - Chemistry for JEE Main & Advanced
| 1. What is a carbocation? |  |
| 2. How is a carbocation formed? |  |
| 3. What are the properties of carbocations? |  |
| 4. What is a carbanion? |  |
| 5. How are carbanions formed? |  |






















