Geometrical Isomerism | Chemistry Class 11 - NEET PDF Download
What is Geometrical Isomerism?
- Isomers that possess the same molecular and structural formula but differ in the arrangement of atoms or groups in space due to restricted rotation are known as geometrical isomers and the phenomenon is known as geometrical isomerism.
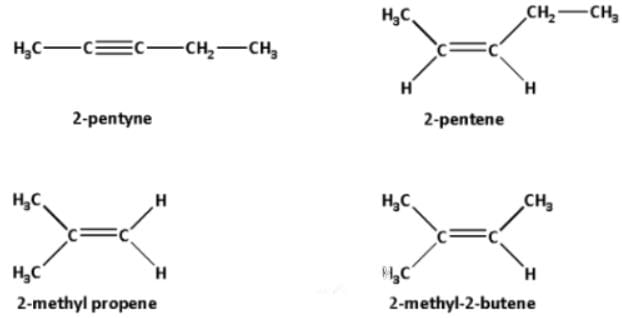
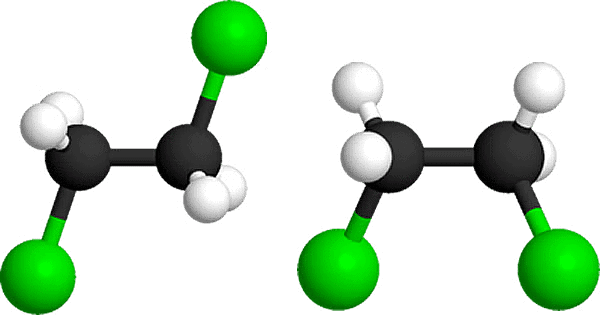 Geometrical Isomers
Geometrical Isomers
Conditions for Geometrical Isomerism
(I) Geometrical isomerism arises due to the presence of a double bond or a ring structure
(i.e. ,
, - N = N - or ring structure).
Due to the rigidity of the double bond or the ring structure to rotate at room temperature, the molecules exist in two or more orientations. This rigidity to rotation is described as restricted rotation l hindered rotation l no rotation.
e.g.
(A) (Restricted Rotation)
(B) (Restricted Rotation)
(II) Different groups should be attached to each doubly bonded atom. For example
and
are identical but not geometrical isomers.
On the other hand, the following types of compounds can exist as geometrical isomers :
,
or
Examples of Geometrical Isomers
(I) Along bond
and
and
(II) Along
and
and
and
and
(III) Along - N = N - bond
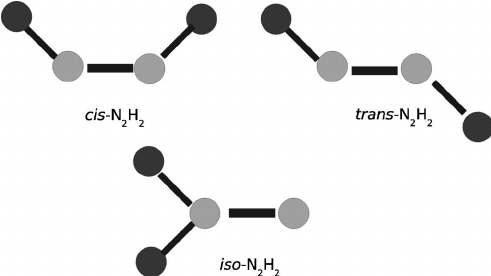 Geometrical Isomers of N2H2
Geometrical Isomers of N2H2
and
and
(IV) Along the bond of cycloalkane
and
and
and
and
(V) Along in ring structures :
Usually in cycloalkenes double bond has its configuration. Their trans isomers do not exist due to large angle strain. But if the ring is large enough a trans stereoisomer is also possible. The smallest trans-cycloalkene that is stable enough to be isolated & stored is trans-cyclooctene.
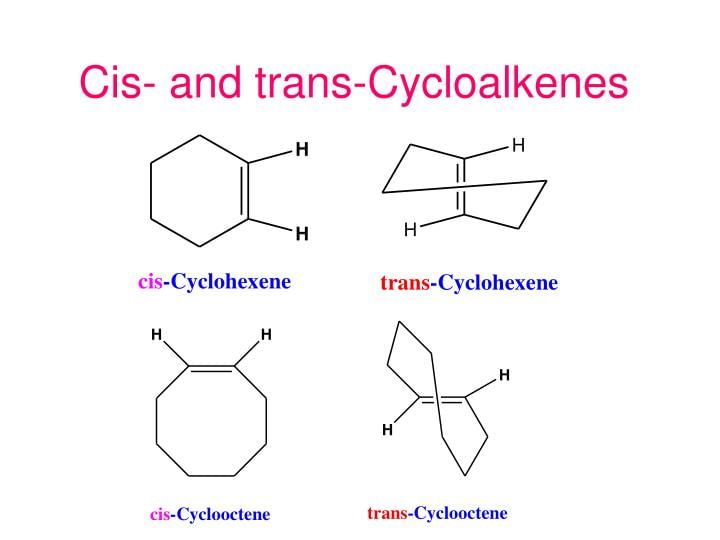 Examples of Cis-Trans Cycloalkenes
Examples of Cis-Trans Cycloalkenes
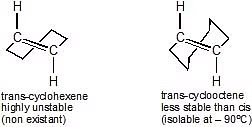 Trans-Cyclohexene and Trans-Cyclooctene
Trans-Cyclohexene and Trans-Cyclooctene
Configurational Nomenclature in Geometrical Isomerism:

For deciding the seniority of groups in E-Z configuration, the Cahn - Ingold - Prelog sequence rules are applied :
Rue I: The group with the first atom having a higher atomic number is senior. According to this rule, the seniority of an atom is :
I > Br > Cl > S > F > O > N > C > H
Rule II: The higher mass isotope is senior.
Thus,
(A) - T > - D > - H.
(B) - C14H3 > - C12H3
Rule III: If the first atom of the group is identical then the second atom is observed for seniority.
e.g.
(A) - CH2Cl > - CH2OH > - CH2NH2 > - CH2 CH3 > - CH3
(B) >
Rule IV: Groups containing double or triple bonds are assigned seniority as if both atoms were duplicated or triplicated that
> C = Y as if it were & - C º Y as if it were
e.g. for deciding seniority among - C º CH, - CH = CH2, their hypothetical equivalents are compared.
>
Rule V: Bond pair gets priority over lone pair.
Rule VI: Z > E & R > S.
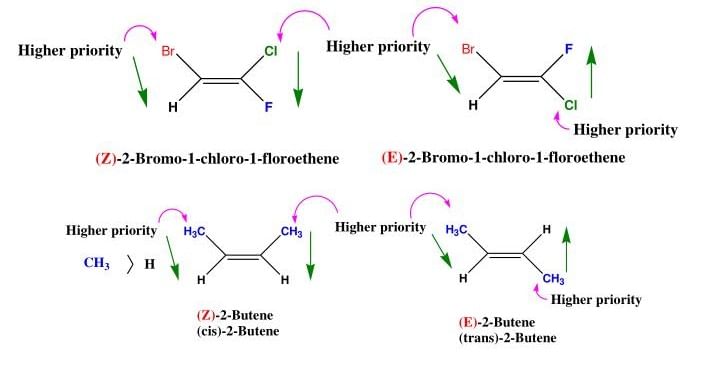
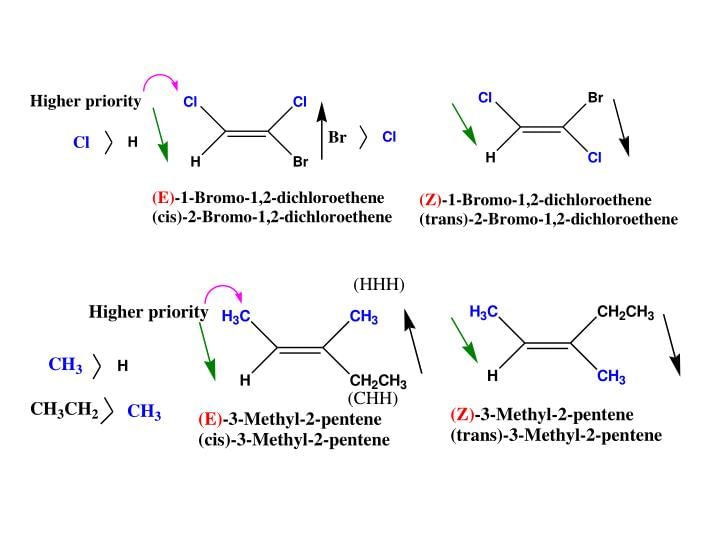 Some Examples of E-Z isomers
Some Examples of E-Z isomers
Number of Geometrical Isomers:
The number of geometrical isomers can be found by calculating the number of stereocentres in the compound. (stereocentre is defined as an atom with three or more different attachments, and interchanging of two of these attachments leads to another stereoisomer. ).
Nature of compound | No. of G.I. (n = no. of stereocentres) | Example | No. of Isomers | Isomers |
(I) Compound with dissimilar ends | 2n | CH3 - CH = CH - CH = CH - C2H5 | 4 | I: (cis, cis) II: (trans, trans) III: (cis, trans) IV: (trans, cis) |
(II) Compound with similar ends with even stereocentres | 2n-1 + 2n/2 - 1 | CH3 - CH = CH - CH = CH - CH3 | 3 | I: (cis, cis) II: (trans, trans) III: (cis, trans) = (trans, cis) |
(III) Compound with similar ends with odd stereocentre |
2n-1 + 2n-1/2 | CH3 – CH = CH – CH = CH – CH = CH – CH3 | 6 | I: (cis, cis, cis) II: (cis, cis, trans) = (trans, cis, cis) III: (cis, trans, trans) = (trans, trans, cis) IV: (trans, trans, trans) V: (cis, trans, cis) VI: (trans, cis, trans) |
Physical Properties of Geometrical Isomers :
Physical properties | I(cis), II(trans) | Remarks |
Dipole moment | I > II | cis-isomer has a resultant dipole moment while in trans-isomer dipole moments cancel out. |
Boiling point | I > II | Molecules having higher dipole moments have higher boiling points due to the larger intermolecular force of attraction. |
Solubility (in H2O) | I > II | More polar molecules are more soluble in H2O. |
Melting point | II > I | More symmetric isomers have higher melting points due to better packing in crystalline lattice & trans isomers are more symmetric than cis. |
Stability | II > I | The molecule having more Vander Waals' strain is less stable. In cis isomer, the bulky groups are closer they have larger Vander Waals' strain. |
|
119 videos|346 docs|74 tests
|
FAQs on Geometrical Isomerism - Chemistry Class 11 - NEET
| 1. What is Geometrical Isomerism? |  |
| 2. What are the conditions for Geometrical Isomerism? |  |
| 3. Can you provide examples of Geometrical Isomers? |  |
| 4. How is configurational nomenclature used in Geometrical Isomerism? |  |
| 5. How many Geometrical Isomers can a molecule have? |  |