Hydrogen: Position of Hydrogen, Isotopes, Properties & Uses | Chemistry for JEE Main & Advanced PDF Download
Position of Hydrogen in the Periodic Table
Hydrogen holds the position as the first element in the periodic table and is recognized as the smallest element in terms of both size and atomic mass.
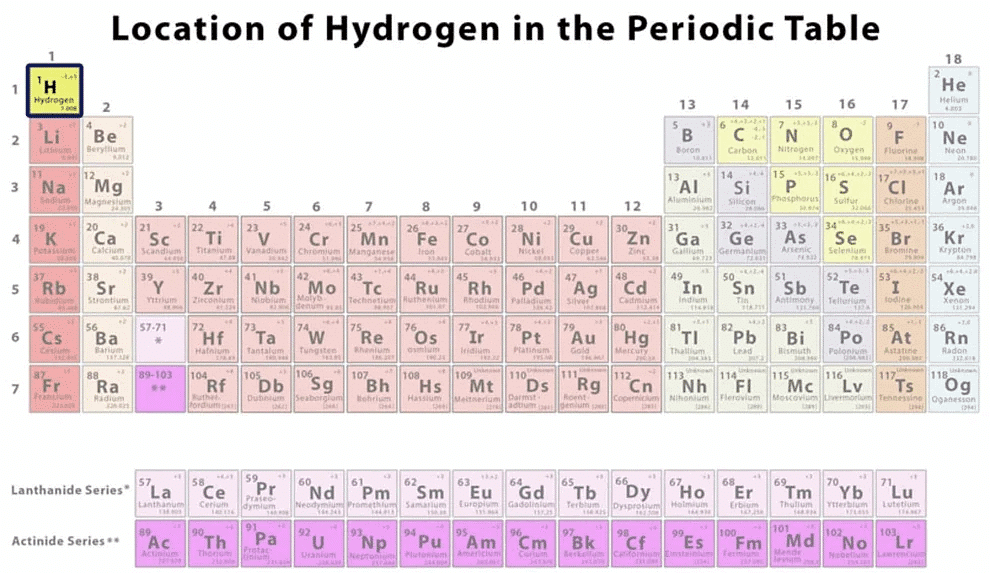 Position of Hydrogen in the Periodic Table
Position of Hydrogen in the Periodic Table
- Having an atomic number of one, hydrogen consists of a single electron in its outermost shell, known as the valence shell. This feature, combined with its relatively low atomic weight, makes hydrogen one of the lightest elements found in the periodic table.
Hydrogen, occupying the initial position on the periodic table, stands as the smallest element with an atomic number of one.
Notably, hydrogen is the lightest element in the periodic table, possessing a single electron in its shell. The electronic configuration of hydrogen, marked by a count of 1, allows it the flexibility to either lose one electron to achieve a noble gas configuration, aligning with the traits of alkali metals, or gain an additional electron, akin to the characteristics of halogens.
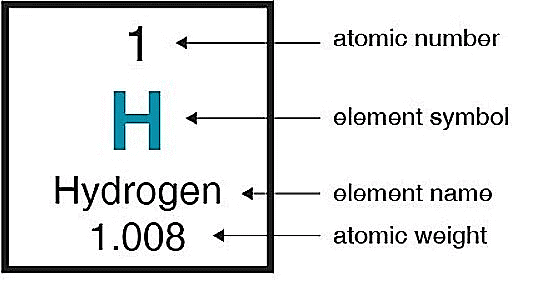 Hydrogen
Hydrogen
Resemblance of Hydrogen with Alkali Metals:
Hydrogen exhibits intriguing similarities with alkali metals in terms of its electronic configuration, reducing properties, and formation of halides. However, distinct characteristics set hydrogen apart and warrant its separate placement in the periodic table.
1. Electronic Configuration:
- Hydrogen has one electron in its valence shell-like alkali metals.

- They seek stability by gaining one electron to achieve a configuration resembling the next noble gas.
- Both hydrogen and alkali metals form unipositive ions.
For example,
Na → Na+ + e–
H → H+ + e–
2. Good Reducing Agent:
- Like alkali metals, hydrogen acts as a strong reducing agent.
- Reducing agents lose or donate electrons in chemical reactions.
3. Formation of Halides:
- Hydrogen, like alkali metals, forms halides.
- It combines with electronegative halogen elements from Group 17 to form compounds such as hydrogen chloride (HCl) and sodium chloride (NaCl).
4. Separate Placement in the Periodic Table:
- Due to distinct characteristics and differences in properties, hydrogen is placed separately from alkali metals.
- It shares similarities not only with alkali metals but also with halogens, making a separate placement more appropriate.
Resemblance of Hydrogen with Halogen
The resemblance between hydrogen and halogens in terms of their electron configuration, chemical properties, and reactivity is intriguing. While hydrogen exhibits unique characteristics, its similarities with halogens contribute to a deeper understanding of its position in the periodic table.1. Electronic Configuration:
- Hydrogen and halogens share similarities in their electron configuration.
- Hydrogen has one electron in its outer shell, while halogens have seven electrons.
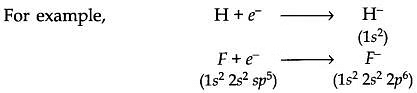
- Both hydrogen and halogens require additional electrons to achieve a stable electron configuration.
2. Electronegative Character:
- Halogens have a strong tendency to gain one electron and form negative ions.
- Similarly, hydrogen has the ability to gain an electron and form hydride ions.
- H+eˉ→Hˉ
Cl+eˉ→Clˉ
3. Ionization Enthalpy:
- Hydrogen's ionization enthalpy is comparable to that of halogens, although higher than that of alkali metals.
4. Oxidation State:
- Hydrogen, like halogens, exhibits an oxidation state of -1.
5. Liberation at the Anode:
- Hydrogen is liberated at the anode during the electrolysis of fused alkali metal hydrides.
- Similarly, halogens are released at the anode during the electrolysis of fused alkali metal halides.
6. Atomicity and Non-Metallic Character:
- Hydrogen, like halogens, exists as a diatomic molecule.
- Both hydrogen and halogens display non-metallic characteristics.
7. Combination with Metals:
- Hydrogen reacts with electropositive alkali and alkaline earth metals to form metallic hydrides.
- Similarly, halogens react with certain metals to form metallic halides.
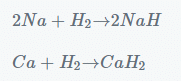
8. Formation of Covalent Compounds:
- Hydrogen readily forms covalent compounds with non-metals such as carbon, silicon, and nitrogen. For example, CH4, SiH4, CCl4, SiCl4
9. Replacement or Substitution Reactions:
- Hydrogen and halogens can be substituted for one another in various carbon-based compounds, resulting in the formation of new compounds.
Occurrence and Isotopes of Hydrogen
Occurrence of Dihydrogen
- Hydrogen is the most common element in the universe, making up 70% of its total mass and being a primary component in the sun. Jupiter and Saturn, the giant planets, are predominantly composed of hydrogen.
- However, on Earth, it is much less common in the atmosphere, making up only 0.15% by mass. Yet, in combined forms like water, it constitutes 15.4% of the Earth's crust and oceans. Hydrogen is found in various compounds in plants, animals, carbohydrates, proteins, and hydrocarbons.
Isotopes of Hydrogen
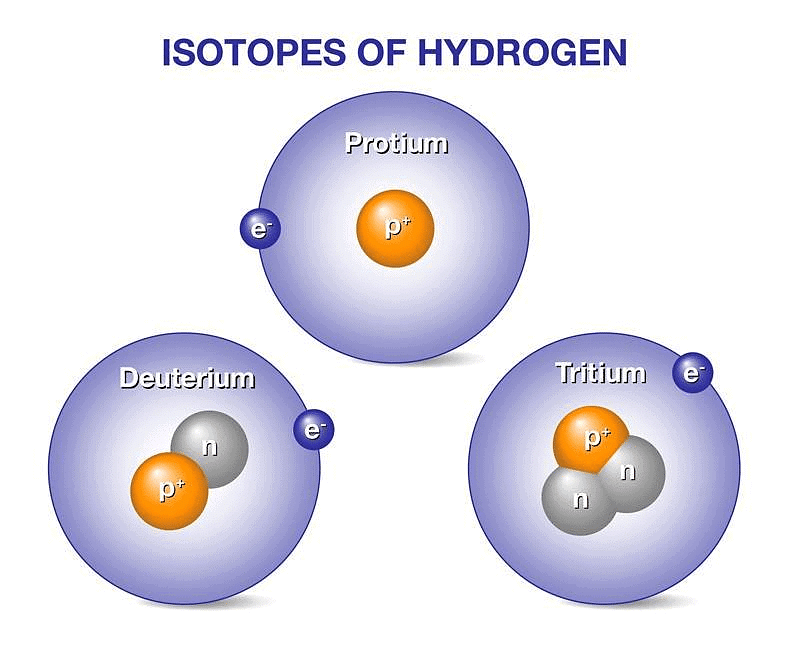
Three naturally existing isotopes of hydrogen are tritium, deuterium, and protium.
 Hydrogen Isotopes
Hydrogen Isotopes
Each isotope comprehends unique properties. These isotopes are commonly used to date. 4H to 7H are nuclei isotopes that are incorporated in the laboratory. One of the least stable isotopes of hydrogen is 7H and the most stable isotope is 5H. The most stable radioisotope of hydrogen is tritium.
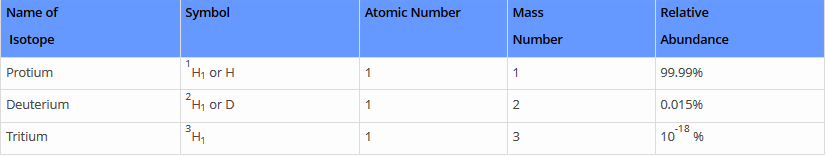
Hydrogen has three isotopes:
- Protium 1H1 or H,
- Deuterium 2H1 or D &
- Tritium 3H1 or T.
These all differ from each other in respect of the number of neutrons present in the nucleus. Protium does not contain any neutron, Deuterium (also known as heavy hydrogen) contains one neutron while the number of neutrons in the nucleus of tritium is 2. Tritium is radioactive and emits low energy β- particles.
 Comparison of Isotopes of Hydrogen
Comparison of Isotopes of Hydrogen
1. Protium (1H )
- It is one of the common isotopes of hydrogen. It is plenty in nature with an abundance of 99.98%.
- One of the reasons for this is that the nucleus of this isotope consists of a single proton and this proton at no time, has been reported to be decayed.
- The mass of protium is 1.007825 amu. Hydrogen generally combines with other atoms in compounds and are usually found in H2 ( diatomic hydrogen gas).
Applications of Protium
1. Hydrogen Fuel Cells
2. Ammonia Production
3. Hydrogenation Reactions
4. Heat Treatment and Annealing
5. Metal Production
6. Oil Refining
7. Balloons and Airships
8. Welding and Cutting
9. Cooling Agent
2. Deuterium (2H)
- It comprises 1 proton and 1 neutron in its nucleus.
- The nucleus of hydrogen 2 is termed as deuteron. It is not radioactive. Its compounds are used in chemical analysis and solvents for hydrogen 1.
- Heavy water is enriched with molecules consisting of deuterium instead of protium. It used as a coolant and a neutron moderator.
- Hydrogen 2 is also used as a fuel in nuclear fusion (commercial). It occurs naturally as deuterium gas.
Applications of Deuterium
- Drugs
- Nuclear weapons
- Contrast properties
- Tracing
- NMR spectroscopy
- Nuclear reactors and Nuclear Power Plants
3. Tritium (3H )
It comprises 2 neutrons and 1 proton in its nucleus. Small traces of hydrogen 3 or tritium occurs in nature due to the synergy of cosmic rays with atmospheric gases. They are also released in a small amount at the time of nuclear weapons tests. It is radioactive, it decays into helium 3 through beta decay. Hydrogen 3 as an atomic mass of 3.0160492 u.
Applications of Tritium
- Analytical chemistry
- Controlled nuclear fusion
- Tritium in hydrogen bomb secondaries
- Boosting
- Neutron initiator
- Nuclear weapons
- Self-powered lighting
- Used as an oceanic transient tracer
4. Hydrogen-4
It comprises 1 proton and 3 neutrons in its nucleus. Hydrogen-4 is a highly unstable isotope of hydrogen. It is incorporated in laboratories bombarding tritium with fast-moving deuterium nuclei. Its atomic mass is 4.02781 ± 0.00011.
5. Hydrogen-5
It comprises 4 neutrons and 1 proton. Hydrogen-5 is a highly unstable isotope of hydrogen. It has been incorporated in the laboratory by bombarding tritium with fast-moving tritium nuclei.
6. Hydrogen-6
It has a half-life of 290 yoctoseconds. It decays through triple neutron emission into hydrogen-3.
7. Hydrogen-7
It comprises 6 neutrons and 1 proton. It has a half-life of 23 yoctoseconds.
Physical Properties of Hydrogen
Hydrogen, as an element, possesses several noteworthy physical properties:
 Physical Properties of Hydrogen
Physical Properties of Hydrogen
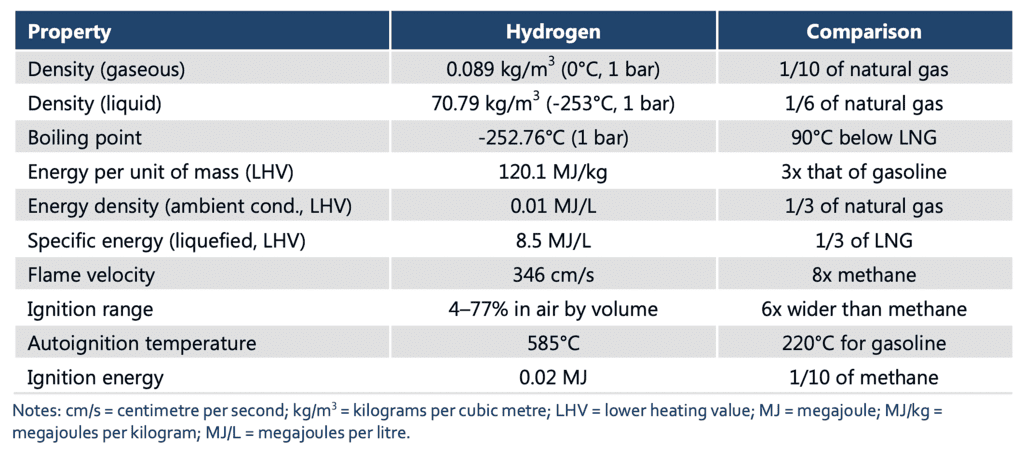
- Molecular Structure: Hydrogen exists as a diatomic molecule, H2, in its most stable form. Each molecule consists of two hydrogen atoms bound together by a covalent bond.
- State of Matter: Hydrogen can exist in all three states of matter. At standard temperature and pressure (STP), hydrogen is a colorless, odorless gas. However, at extremely low temperatures near absolute zero (-273.15°C or -459.67°F), it can condense into a liquid. Under high pressure, hydrogen can solidify into a crystalline structure.
- Density: Hydrogen gas has a very low density, making it lighter than air. It is the least dense element on Earth. This characteristic contributes to its tendency to rise and disperse rapidly when released into the atmosphere.
- Boiling and Melting Points: Hydrogen has low boiling and melting points. At STP, hydrogen boils at -252.87°C (-423.17°F) and melts at -259.16°C (-434.49°F). These extremely low temperatures are due to the weak intermolecular forces between hydrogen molecules.
- Solubility: Hydrogen gas is relatively insoluble in most solvents, including water. It has low solubility in liquids, and its solubility decreases with increasing temperature.
- Color and Transparency: Hydrogen gas is colorless, indicating that it does not absorb or emit visible light. It is transparent, allowing light to pass through it without significant absorption or scattering.
- Compressibility: Hydrogen gas is highly compressible. Under increasing pressure, hydrogen molecules can be compressed into a smaller volume, resulting in a higher density.
- Thermal Conductivity: Hydrogen gas exhibits relatively high thermal conductivity. It can efficiently transfer heat energy due to the rapid motion of its molecules.
These physical properties of hydrogen contribute to its unique behavior and applications in various scientific, industrial, and energy-related fields.
Chemical Properties of Hydrogen
Hydrogen, as an element, displays various chemical properties that contribute to its reactivity and versatility:
 Chemical Properties of Hydrogen
Chemical Properties of Hydrogen
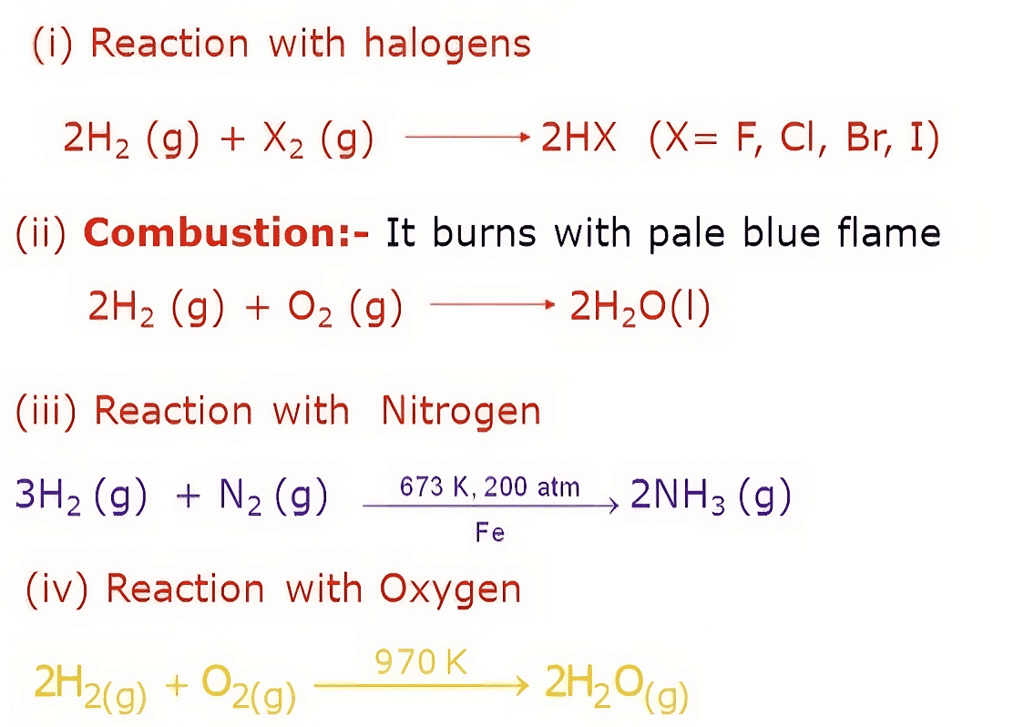
- Combustibility: Hydrogen is highly flammable and readily combusts in the presence of an oxidizing agent, such as oxygen. When ignited, hydrogen reacts with oxygen in a rapid and exothermic combustion reaction, releasing energy and producing water vapor as a byproduct.
- Reducing Agent: Hydrogen acts as a powerful reducing agent, meaning it has a tendency to donate electrons in chemical reactions. It can react with various compounds, including metal oxides, to reduce them and form elemental metals.
- Reactivity with Halogens: Hydrogen reacts with halogens (Group 17 elements) to form hydrogen halides. For example, hydrogen combines with chlorine to produce hydrogen chloride (HCl) and with bromine to form hydrogen bromide (HBr).
- Formation of Hydrides: Hydrogen can react with many elements to form compounds called hydrides. Metallic hydrides, such as lithium hydride (LiH) or sodium hydride (NaH), are formed by the reaction of hydrogen with alkali metals. Covalent hydrides, such as methane (CH4) and ammonia (NH3), are formed by the reaction of hydrogen with non-metals.
- Reactivity with Carbon: Hydrogen forms hydrocarbons when combined with carbon. This reaction is the basis for organic chemistry, as hydrogen can bond with carbon atoms to form a wide range of organic compounds.
- Isotope Exchange: Hydrogen can undergo isotope exchange reactions, where the hydrogen atoms are replaced with different isotopes of hydrogen, such as deuterium (heavy hydrogen) or tritium (radioactive hydrogen). These reactions have applications in various scientific and industrial fields.
- Acid-Base Reactions: Hydrogen ions (H+) can act as acids by donating protons in acid-base reactions. Hydrogen gas can dissolve in water, producing hydronium ions (H3O+), which contribute to the acidity of the solution.
- Reactivity with Electropositive Metals: Hydrogen can react with some metals, particularly at high temperatures, to form metal hydrides. These reactions are utilized in hydrogen storage and purification processes.
These chemical properties of hydrogen make it a versatile element with a wide range of applications, from energy production to chemical synthesis and various industrial processes.
Uses of Hydrogen
Uses of Hydrogen are as follows: Uses of Hydrogen
Uses of Hydrogen
- Commercial fixation of nitrogen from the air in the Haber ammonia process.
- Hydrogenation of fats and oils.
- Hydrogen use in vehicles.
- Methanol production, in hydrodealkylation, hydrocracking, and hydrodesulfurization.
- Hydrogen fuel cells produce electricity.
- Rocket fuel is a major use of hydrogen for energy.
- Welding.
- Hydrogen is used in the synthesis of ammonia and the manufacture of nitrogenous fertilizers.
- Hydrogenation of unsaturated vegetable oils for manufacturing vanaspati fat.
- Production of hydrochloric acid.
- Reduction of metallic ores.
|
334 videos|651 docs|300 tests
|
FAQs on Hydrogen: Position of Hydrogen, Isotopes, Properties & Uses - Chemistry for JEE Main & Advanced
| 1. What is the position of hydrogen in the periodic table? |  |
| 2. How does hydrogen resemble alkali metals? |  |
| 3. What is the resemblance between hydrogen and halogens? |  |
| 4. What are the isotopes of hydrogen? |  |
| 5. What are the physical properties of hydrogen? |  |

















