Hydrogen Spectrum Series | Physics for JEE Main & Advanced PDF Download
Hydrogen Spectrum Series
Each element emits a spectrum of radiation, which is characteristic of the element itself. The spectrum consists of a set of isolated parallel lines and is called the line spectrum.
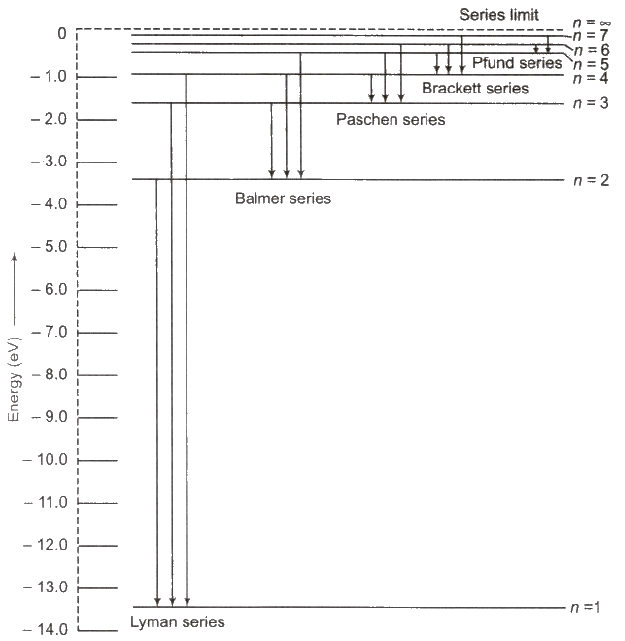
Hydrogen spectrum contains five series
(i) Lyman Series When electron jumps from n = 2, 3,4, …orbit to n = 1 orbit, then a line of Lyman series is obtained.
This series lies in ultra violet region.
(ii) Balmer Series When electron jumps from n = 3, 4, 5,… orbit to n = 2 orbit, then a line of Balmer series is obtained.
This series lies in visual region.
(iii) Paschen Series When electron jumps from n = 4, 5, 6,… orbit to n = 3 orbit, then a line of Paschen series is obtained.
This series lies in infrared region
(iv) Brackett Series When electron jumps from n = 5,6, 7…. orbit to n = 4 orbit, then a line of Brackett series is obtained.
This series lies in infrared region.
(v) Pfund Series When electron jumps from n = 6,7,8, … orbit to n = 5 orbit, then a line of Pfund series is obtained.
This series lies in infrared region.
Wave Model
It is based on wave mechanics. Quantum numbers are the numbers required to completely specify the state of the electrons.
In the presence of strong magnetic field, the four quantum number are
(i) Principle quantum number (n) can have value 1,2, … ∞
(ii) Orbital angular momentum quantum number l can have value 0,1, 2, … ,(n – 1).
(iii) Magnetic quantum number (me) which can have values – I to I.
(iv) Magnetic spin angular momentum quantum number (ms) which can have only two value + 1 / 2.
|
268 videos|756 docs|171 tests
|
FAQs on Hydrogen Spectrum Series - Physics for JEE Main & Advanced
| 1. What is the hydrogen spectrum series? |  |
| 2. How is the hydrogen spectrum series useful in scientific research? |  |
| 3. What are the different series in the hydrogen spectrum? |  |
| 4. What is the significance of the Balmer series in the hydrogen spectrum? |  |
| 5. How does the hydrogen spectrum series contribute to our understanding of atomic structure? |  |

















