Test: Chemistry - 2 - UPSC MCQ
20 Questions MCQ Test - Test: Chemistry - 2
Match List-I with List-II and select the correct answer from the codes given below:




. Match List-I with List-II and select the correct answer from the codes given below:




When soggy biscuits are kept inside the fridge for sometime they become crisp because
Which one of the following statements is not true about cosmic rays?
In cold weather, aquatic animals survive even when water to the top layer of the lake freezes into ice because
Consider the following statements:
Assertion (A): LPG is a pollution free vehicular fuel.
Reason (R): Plying of CNG fuelled-buses is recommended for metropolitan cities in India.
Match List-I with List-II and select the correct answer from the codes given below:


Match List-I with List-II and select the correct answer from the codes given below:
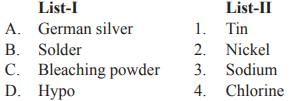

Consider the following statements: Hard water is not suitable for
1. Drinking
2. Washing clothes with soap
3. Use in boilers
4. Irrigating crops
Q. Which of these statements are correct?
Match List-I with List-II and select the correct answer from the codes given below:


What is the role of ultraviolet (UV) radiation in the water purification system?
1. It inactivates / kills the harmful microorganisms in water.
2. It removes all the undesirable odours from the water.
3. It quickens the sedimentation of solid particles and improves the clarity of water.
Q. Which of the statements given above is/are correct?
Which one of the following sets of elements was primarily responsible for the origin of life on the Earth?
Acid rains is caused due to emission of which of the following into the atmosphere?
A sample of chloroform before using as an anaesthetic, is tested by














