Test: Class 10 General Science NCERT Based - 1 - UPSC MCQ
15 Questions MCQ Test - Test: Class 10 General Science NCERT Based - 1
Regarding the Chemical reactions, consider the following statements:
1. New atoms are produced in a chemical reaction.
2. Exothermic reactions are accompanied by absorption of Heat.
Which of the statements given above is/are correct?
Which of the following best defines the oxidation of any substance?
Regarding the oxidation, consider the following statements:
1. The Black coating on silver and the green coating on copper are examples of Corrosion.
2. Bags of chips are flushed with gas such as nitrogen to prevent the chips from getting oxidised.
Which of the statements given above is/are correct?
Regarding the lime, consider the following statements:
1. Calcium hydroxide is also called as quicklime.
2. Calcium hydroxide reacts slowly with the carbon dioxide in air to form a thin layer of calcium carbonate on the walls after two to three days of whitewashing.
Which of the statements given above is/are correct?
Respiration is the type of which of the following reactions?
The neutralization reaction between an acid and a base is a type of:
Which of the following gases is used in the storage of fat and oil containing foods for a long time?
The respiration process during which glucose undergoes slow combustion by combining with oxygen in the cells of our body to produce energy, is a kind of:
A chemical reaction does not involve:
Consider the following pairs:
Match-I (Homogeneous mixture)
1. Mercury and any other metal
2. Copper and Zinc
3. Copper and tin
4. Lead and Tin
Match-II (Alloy)
Amalgam
Brass
Bronze
Solder
Which of the following pairs given above is/are correctly matched?
Regarding a 'human stomach', consider the following statements:
1. Human stomach produces sulphuric acid that helps in the digestion of food without harming the stomach.
2. During indigestion, the stomach produces too much acid and this causes pain and irritation.
Which of the statements given above is/are correct?
Consider the following statements:
1. The human body works within the pH range of 7.0 to 7.8.
2. When pH of rain water is less than 5.6, it is called acid rain.
3. Tooth decay starts when the pH value of the mouth increases.
Which of the statements given above is/are correct?
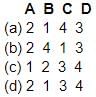
Match List-I with List-II and using the code given below, select the correct answer:
List-I (matter)
A. Human blood
B. Milk
C. Human saliva
D. Human Urine
List-II (pH value)
1. 5.5 to 7.5
2. 7.3 to 7.5
3. 6.4
4. 6.5 to 7.5
Code:

Match List-I with List-II and using the code given below, select the correct answer:
List I (Natural source)
A. Bee sting
B. Tamarind
C. Tomato
D. Vinegar
List II (Acid)
1. Tartaric acid
2. Methanoic acid
3. Acetic acid
4. Oxalic acid
Which of the following is used in a soda-acid fire extinguisher?














