UPSC Exam > UPSC Tests > Test: Materials: Metals & Non Metals- 2 - UPSC MCQ
Test: Materials: Metals & Non Metals- 2 - UPSC MCQ
Test Description
20 Questions MCQ Test - Test: Materials: Metals & Non Metals- 2
Test: Materials: Metals & Non Metals- 2 for UPSC 2025 is part of UPSC preparation. The Test: Materials: Metals & Non Metals- 2 questions and answers have been prepared
according to the UPSC exam syllabus.The Test: Materials: Metals & Non Metals- 2 MCQs are made for UPSC 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Materials: Metals & Non Metals- 2 below.
Solutions of Test: Materials: Metals & Non Metals- 2 questions in English are available as part of our course for UPSC & Test: Materials: Metals & Non Metals- 2 solutions in
Hindi for UPSC course.
Download more important topics, notes, lectures and mock test series for UPSC Exam by signing up for free. Attempt Test: Materials: Metals & Non Metals- 2 | 20 questions in 20 minutes | Mock test for UPSC preparation | Free important questions MCQ to study for UPSC Exam | Download free PDF with solutions
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 1
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 2
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 3
Test: Materials: Metals & Non Metals- 2 - Question 4
The metal which can form a barrier layer of oxide on its surface is :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 4
Test: Materials: Metals & Non Metals- 2 - Question 5
Metal are hard but _____ can be cut with a knife
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 5
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 6
Test: Materials: Metals & Non Metals- 2 - Question 7
Metal oxides react with water to produce :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 7
Test: Materials: Metals & Non Metals- 2 - Question 8
If metallic Zinc is added to copper sulphate solution :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 8
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 9
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 10
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 11
Test: Materials: Metals & Non Metals- 2 - Question 12
Metals that react with both acids and bases are called :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 12
Test: Materials: Metals & Non Metals- 2 - Question 13
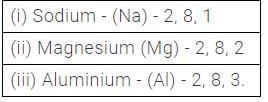
How many electrons are generally present in metals in their valence shell?
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 13
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 14
Test: Materials: Metals & Non Metals- 2 - Question 15
Zinc reacts with hydrochloric acid to form :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 15
Test: Materials: Metals & Non Metals- 2 - Question 16
When Silver is added to Copper Nitrate solution result is :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 16
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 17
Test: Materials: Metals & Non Metals- 2 - Question 18
That property of a metal by virtue of which it can be drawn into wires is
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 18
Test: Materials: Metals & Non Metals- 2 - Question 19
The metal alloyed with gold for making jewellery :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 19
Test: Materials: Metals & Non Metals- 2 - Question 20
Gas evolved when Calcium oxide reacts with water is/are :
Detailed Solution for Test: Materials: Metals & Non Metals- 2 - Question 20
Information about Test: Materials: Metals & Non Metals- 2 Page
In this test you can find the Exam questions for Test: Materials: Metals & Non Metals- 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Materials: Metals & Non Metals- 2, EduRev gives you an ample number of Online tests for practice
Download as PDF