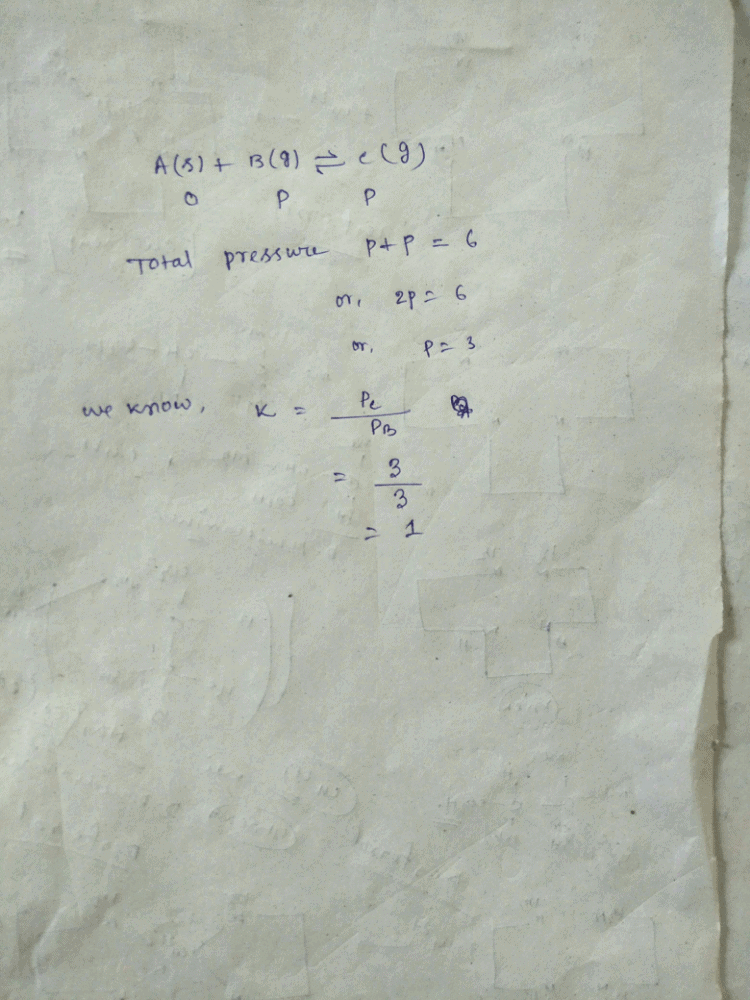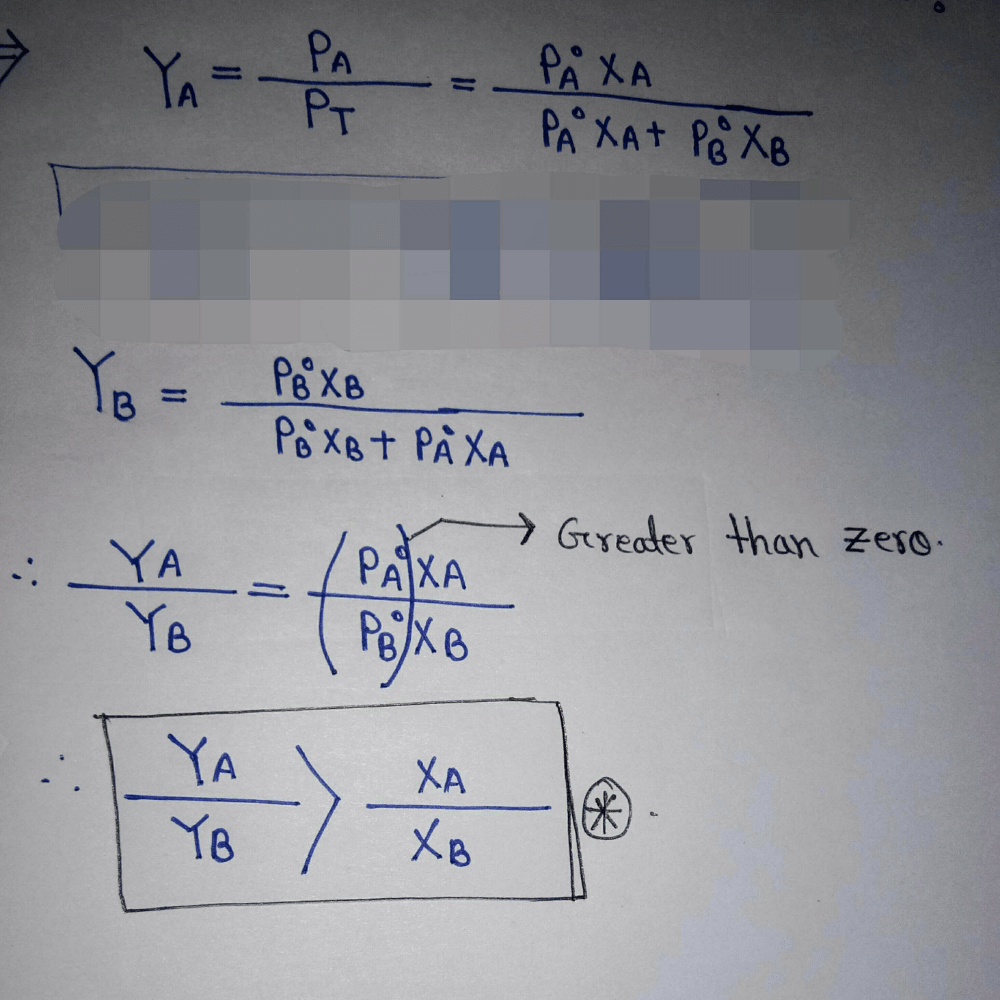|
|
Nepal DeyI love chemistry and I want to get admission in IIT |

|
Nepal Dey
EduRev Chemistry
|
Content, tests & courses saved by you for accessing later. (visible to you only)
Top Scoring Tests by Nepal
Test: Adsorption - 1 | 37/40 |
Test: Conductance - 1 | 33/40 |
Test: Solid State - 1 | 33/40 |
Test: Main Group- 3 | 47/60 |
Test: Gaseous State - 1 | 30/40 |
Test: Colligative Properties - 2 | 43/60 |
Test: Electrochemistry - 2 | 44/62 |
Test: Electrochemistry - 3 | 43/60 |
Test: Main Group - 2 | 28/40 |
Test: GOC, Aromaticity, Acidity & Basicity - 1 | 27/40 |
Discussed Questions

|
Nepal Dey upvoted • Dec 10, 2020 |
Which of the following compounds have higher enol content?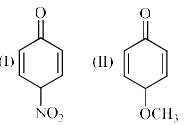
- a)I
- b)II
- c)I = II
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds have higher enol content?
a)
I
b)
II
c)
I = II
d)
None of these

|
Naveen Dahiya answered |
I think answer will be A bcz for enol content O is replaced by OH here H comes from No2 carbon sites which is highly stable .
Increasing order of pKa values (pKa = –log Ka) of H2O, CH3OH and C6H5OH is:
- a) H2O < CH3OH < C6H5OH
- b)CH3OH < H2O < C6H5OH
- c)C6H5OH < CH3OH < H2O
- d) C6H5OH < H2O < CH3OH
Correct answer is option 'D'. Can you explain this answer?
Increasing order of pKa values (pKa = –log Ka) of H2O, CH3OH and C6H5OH is:
a)
H2O < CH3OH < C6H5OH
b)
CH3OH < H2O < C6H5OH
c)
C6H5OH < CH3OH < H2O
d)
C6H5OH < H2O < CH3OH

|
Nepal Dey answered • Dec 10, 2020 |
Correct ans 100% sure C. pka value of c6h5oh =10 < />
ch3oh=15.5 < h20="15.7" h20="" />
ch3oh=15.5 < h20="15.7" h20="" />

|
Nepal Dey upvoted • Dec 10, 2020 |
Increasing order of pKa values (pKa = –log Ka) of H2O, CH3OH and C6H5OH is:
- a) H2O < CH3OH < C6H5OH
- b)CH3OH < H2O < C6H5OH
- c)C6H5OH < CH3OH < H2O
- d) C6H5OH < H2O < CH3OH
Correct answer is option 'D'. Can you explain this answer?
Increasing order of pKa values (pKa = –log Ka) of H2O, CH3OH and C6H5OH is:
a)
H2O < CH3OH < C6H5OH
b)
CH3OH < H2O < C6H5OH
c)
C6H5OH < CH3OH < H2O
d)
C6H5OH < H2O < CH3OH
|
|
Sandeep Sahoo answered |
The correct answer should be C. As phenol is more acidic than methanol then water.
Correct statement on the effect of addition of aq. HCl on the equilibrium is: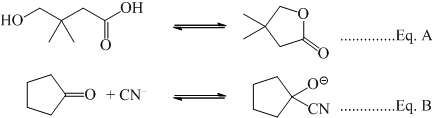
- a)Equilibrium will shift towards right in case of both A and B
- b)Equilibrium will shift towards left in case of both A and B
- c)Equilibrium will shift towards right in A and left in case of B
- d)Equilibrium will shift towards right in B and left in case of A
Correct answer is option 'A'. Can you explain this answer?
Correct statement on the effect of addition of aq. HCl on the equilibrium is:
a)
Equilibrium will shift towards right in case of both A and B
b)
Equilibrium will shift towards left in case of both A and B
c)
Equilibrium will shift towards right in A and left in case of B
d)
Equilibrium will shift towards right in B and left in case of A

|
Nepal Dey answered • Dec 07, 2020 |
Because hcl increase the reactivity of the both reactant i. e. increase the reactivity of ketone center
9.2 grams of N2O4(g) is taken in a closed on litre vessel and heated till the following equilibrium is reached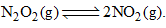 At equilibrium, 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre–1) (molecular weight of N2O4 = 92)
At equilibrium, 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre–1) (molecular weight of N2O4 = 92)- a)0.1
- b)0.4
- c)0.2
- d)2
Correct answer is option 'C'. Can you explain this answer?
9.2 grams of N2O4(g) is taken in a closed on litre vessel and heated till the following equilibrium is reached
At equilibrium, 50% N2O4(g) is dissociated. What is the equilibrium constant (in mol litre–1) (molecular weight of N2O4 = 92)
a)
0.1
b)
0.4
c)
0.2
d)
2

|
Nepal Dey answered • Dec 07, 2020 |
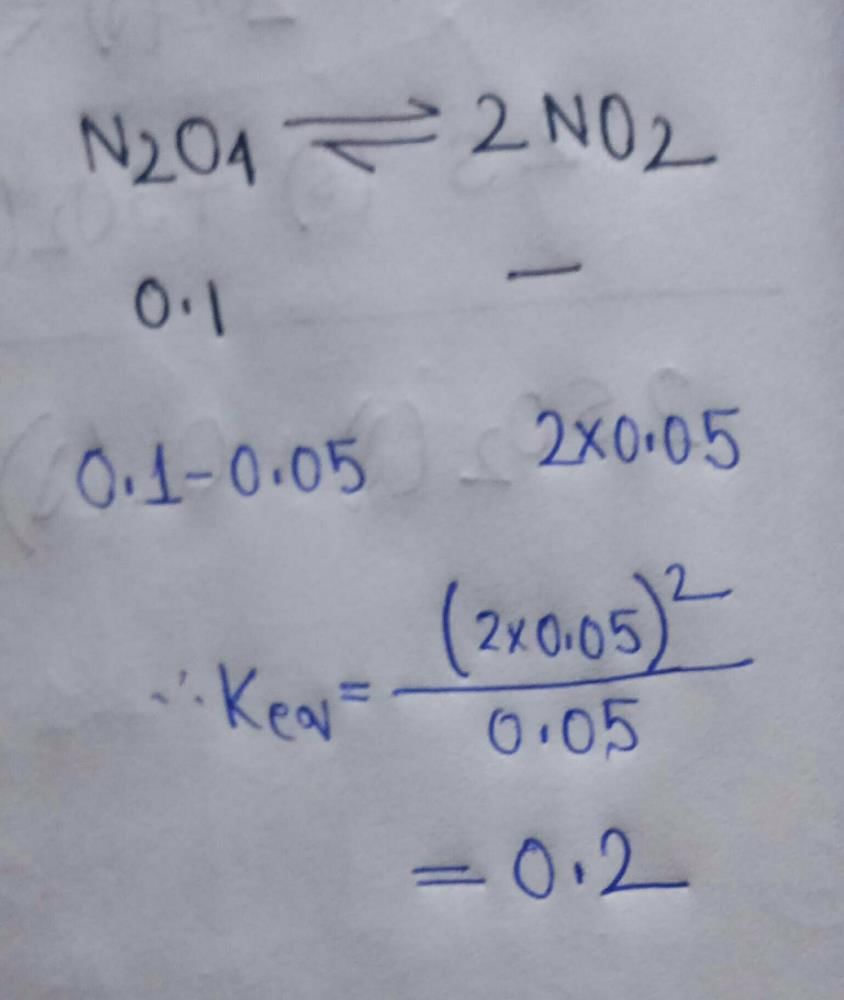
At a certain temperature, the following reactions have the equilibrium constants as shown below:
S(s) + O2(g) <=> SO2(g); Kc1 = 5 X 1052
2S(s) + 3O2(g) <=> 2SO3(g) ; Kc2 = 1029
What is the equilibrium constant, Kc for the reaction at the same temperature?
2SO2(g) + O2(g) <=> 2SO3(g)
... more
At a certain temperature, the following reactions have the equilibrium constants as shown below:
S(s) + O2(g) <=> SO2(g); Kc1 = 5 X 1052
2S(s) + 3O2(g) <=> 2SO3(g) ; Kc2 = 1029
What is the equilibrium constant, Kc for the reaction at the same temperature?
2SO2(g) + O2(g) <=> 2SO3(g)

|
Nepal Dey answered • Dec 07, 2020 |

|
Nepal Dey upvoted • Nov 08, 2020 |
Adsorption of ethanoic acid on wood charcoal follows Freundlich isotherm. Calculate the mass of ethanoic acid adsorbed by 500 g of wood charcoal at 300 K form 3 litre of 0.65 M ethanol solution. The value of constant k = 0.16 and n = 2.35. Also report the molarity of left ethanol in solution.
Correct answer is '17'. Can you explain this answer?
Adsorption of ethanoic acid on wood charcoal follows Freundlich isotherm. Calculate the mass of ethanoic acid adsorbed by 500 g of wood charcoal at 300 K form 3 litre of 0.65 M ethanol solution. The value of constant k = 0.16 and n = 2.35. Also report the molarity of left ethanol in solution.

|
Komal Mavi answered |
THE ANSWER GIVEN IN THE ANSWER KEY IS NOT 17 BUT .17 KINDLY RECHECK FIRST .
Acc to Freundlich adsorption isotherm,
x/m = k.c^1/n
x/500 = 0.16 (0.65)^1/2.35
On solving,
*x = 66.6g*
Initially, we had 3L of 0.65 M ethanol solution
i.e. 0.65 × 3 mol = 1.95 mol = 1.95 ×46 g = 89.7 g of ethanol
Therefore, after adsorption, mass of ethanol in solution = 89.7 g - 66.6 g
= 23.1 g
= 23.1/46 mol = 0.5... more

|
Nepal Dey upvoted • Nov 08, 2020 |
20% surface sites have absorbed N2. On heating N2 gas is evolved from sites and were collected at 0.001 atm and 298 K in a container of volume 2.46 cm3. Density of surface sites is 6.023 × 1014 cm–2 and surface area is 1000 cm2. Find out the number of surface sites occupied per molecule of N2.
Correct answer is '2'. Can you explain this answer?
20% surface sites have absorbed N2. On heating N2 gas is evolved from sites and were collected at 0.001 atm and 298 K in a container of volume 2.46 cm3. Density of surface sites is 6.023 × 1014 cm–2 and surface area is 1000 cm2. Find out the number of surface sites occupied per molecule of N2.

|
Mrinalini Sen answered |
No. of surface sites = 6.023*10^14 * 1000
occupied sites = 20/100 * 6.023 * 10^14 * 1000 = 6.022/5*10^17
no. of moles of N2 = 0.001 * 0.00246 / 0.0821 * 298 = 10^-7
no. of molecules = 10^-7 * 6.022*10^23 = 6.022 * 10^16
so no. of surface sites per molecule of N2 = (6.022*10^17/5)/(6.022*10^16) = 2
Calculate the surface area of a catalyst that adsorbs 103 cm3 of nitrogen reduced to STP per gram in order to form the monolayer. The effective area occupied by N2 molecule on the surface is 1.62 × 10–15 cm2.
Correct answer is '43416000'. Can you explain this answer?
Calculate the surface area of a catalyst that adsorbs 103 cm3 of nitrogen reduced to STP per gram in order to form the monolayer. The effective area occupied by N2 molecule on the surface is 1.62 × 10–15 cm2.

|
Nepal Dey answered • Nov 08, 2020 |

For the reaction, 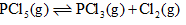 The forward reaction at constant temperature is favoured by
The forward reaction at constant temperature is favoured by
(I) Introducing an inert gas at constant volume
(II) Introducing chlorine gas at constant volume
(III) Introducing an inert gas at constant pressure
(IV) Increasing the volume of the container
(V) Introducing PCl5 at constant volume- a) III only
- b)III and IV
- c)II and V
- d)III and V
Correct answer is option 'A'. Can you explain this answer?
For the reaction, 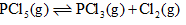
The forward reaction at constant temperature is favoured by
(I) Introducing an inert gas at constant volume
(II) Introducing chlorine gas at constant volume
(III) Introducing an inert gas at constant pressure
(IV) Increasing the volume of the container
(V) Introducing PCl5 at constant volume
(I) Introducing an inert gas at constant volume
(II) Introducing chlorine gas at constant volume
(III) Introducing an inert gas at constant pressure
(IV) Increasing the volume of the container
(V) Introducing PCl5 at constant volume
a)
III only
b)
III and IV
c)
II and V
d)
III and V

|
Nepal Dey answered • Jul 29, 2020 |
 so I think B is the right answer
Two liquids A and B have  in the ratio of 1:3 and the ratio of number of moles of A and B in liquid phase are 1:3 then mole fraction of ‘A’ in vapour phase in equilibrium with the solution is equal to:
in the ratio of 1:3 and the ratio of number of moles of A and B in liquid phase are 1:3 then mole fraction of ‘A’ in vapour phase in equilibrium with the solution is equal to:- a)0.1
- b)0.2
- c)0.5
- d)1.0
Correct answer is option 'A'. Can you explain this answer?
Two liquids A and B have  in the ratio of 1:3 and the ratio of number of moles of A and B in liquid phase are 1:3 then mole fraction of ‘A’ in vapour phase in equilibrium with the solution is equal to:
in the ratio of 1:3 and the ratio of number of moles of A and B in liquid phase are 1:3 then mole fraction of ‘A’ in vapour phase in equilibrium with the solution is equal to:
a)
0.1
b)
0.2
c)
0.5
d)
1.0

|
Nepal Dey answered • Jul 03, 2020 |
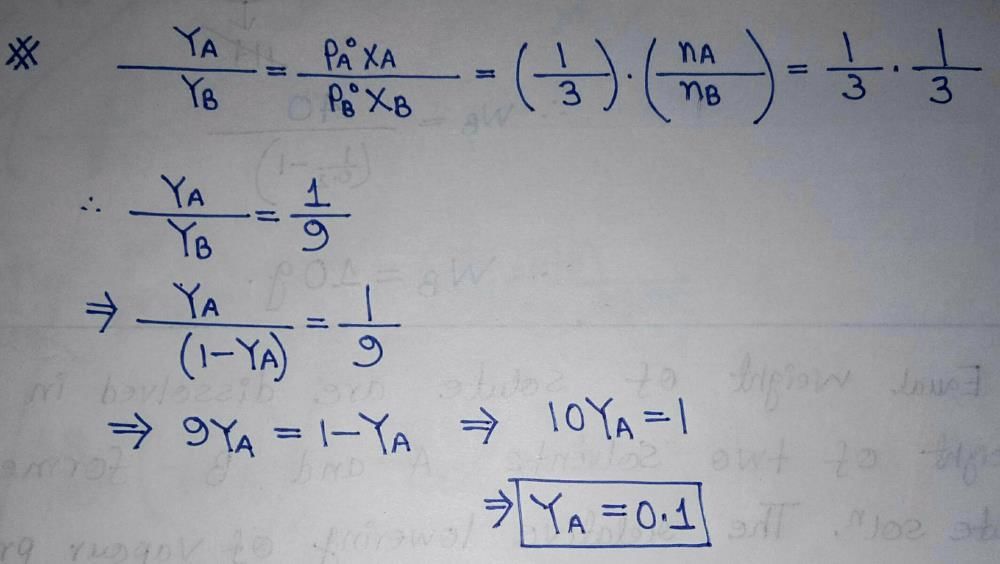

|
Nepal Dey asked • Jan 27, 2020 |
Specific conductance of 0.01 M KCl solution is x ohm–1 cm–1. When conductivity cell is filled with 0.01 M KCl the conductance observed is y ohm–1. When the same cell is filled with 0.01 M H2SO4, the observed conductance was Z ohm–1 cm–1. Hence specific conductance of 0.01 M H2SO4 is:- a)xz
- b)z/xy
- c)xz/y
- d)xy/z
Correct answer is option 'C'. Can you explain this answer?
Specific conductance of 0.01 M KCl solution is x ohm–1 cm–1. When conductivity cell is filled with 0.01 M KCl the conductance observed is y ohm–1. When the same cell is filled with 0.01 M H2SO4, the observed conductance was Z ohm–1 cm–1. Hence specific conductance of 0.01 M H2SO4 is:
a)
xz
b)
z/xy
c)
xz/y
d)
xy/z

|
Maitri Sen answered |
To find the specific conductance of 0.01 M H2SO4 solution, we can use the concept of specific conductance and conductance.
Given:
Specific conductance of 0.01 M KCl solution = x ohm^-1 cm^-1
Conductance observed with 0.01 M KCl in the conductivity cell = y ohm^-1
Conductance observed with 0.01 M H2SO4 in the same conductivity cell = z ohm^-1 cm^-1
The specific ... more
Given:
Specific conductance of 0.01 M KCl solution = x ohm^-1 cm^-1
Conductance observed with 0.01 M KCl in the conductivity cell = y ohm^-1
Conductance observed with 0.01 M H2SO4 in the same conductivity cell = z ohm^-1 cm^-1
The specific ... more

|
Nepal Dey asked • Jan 27, 2020 |
Which one of the following solutions has lowest conducting power:
- a)0.1 M CH3COOH
- b)0.1 M NaCl
- c)0.1 M KNO3
- d)0.1 M HCl
Correct answer is option 'A'. Can you explain this answer?
Which one of the following solutions has lowest conducting power:
a)
0.1 M CH3COOH
b)
0.1 M NaCl
c)
0.1 M KNO3
d)
0.1 M HCl

|
Aryan Choudhary answered |
**Answer:**
To determine the solution with the lowest conducting power, we need to consider the dissociation of the solutes in water. The greater the degree of dissociation, the higher the conducting power of the solution.
**Dissociation of the solutes:**
a) CH3COOH (acetic acid):
CH3COOH ⇌ CH3COO- + H+
b) NaCl (sodium chloride):
NaCl ⇌ Na+ + C... more
To determine the solution with the lowest conducting power, we need to consider the dissociation of the solutes in water. The greater the degree of dissociation, the higher the conducting power of the solution.
**Dissociation of the solutes:**
a) CH3COOH (acetic acid):
CH3COOH ⇌ CH3COO- + H+
b) NaCl (sodium chloride):
NaCl ⇌ Na+ + C... more
Fetching relevant content for you