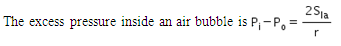All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of November Week 1 for NEET Exam
Which is chlorate (I) ion?
a) 
b)
c)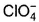
d)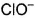
Correct answer is option 'B'. Can you explain this answer?
|
|
Pooja Shah answered |
- ClO3- : A very reactive inorganic anion.
- The term chlorate can also be used to describe any compound containing the chlorate ion, normally chlorate salts.
- Example: Potassium chlorate, KClO3
The difference in the oxidation number of the two types of sulphur atoms in Na2S4O6 is .........[HTJEE2011]
Correct answer is '7'. Can you explain this answer?
The difference in the oxidation number of the two types of sulphur atoms in Na2S4O6 is .........
[HTJEE2011]
|
|
Hrishikesh Sengupta answered |
Difference in oxidation number = 6 - (-1) = 7
Water rises to a height of 20 mm in a capillary. If the radius of the capillary is made 1/3 rd of its previous value, to what height will the water now rise in the tube?- a)60 mm
- b)80 mm
- c)40 mm
- d)30 mm
Correct answer is option 'A'. Can you explain this answer?
Water rises to a height of 20 mm in a capillary. If the radius of the capillary is made 1/3 rd of its previous value, to what height will the water now rise in the tube?
a)
60 mm
b)
80 mm
c)
40 mm
d)
30 mm
|
|
Pooja Mehta answered |
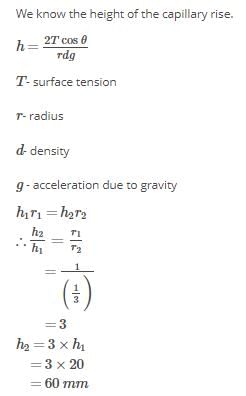
HENCE,CORRECT OPTION IS (A).
Which of the following is not an example of redox reaction?- a)BaCl2 + H2SO4 ⎯→ BaSO4 + 2HCl
- b)Fe2O3 + 3CO ⎯→ 2Fe + 3CO2
- c)2K + F2 ⎯→ 2KF
- d)CuO + H2 ⎯→ Cu + H2O
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not an example of redox reaction?
a)
BaCl2 + H2SO4 ⎯→ BaSO4 + 2HCl
b)
Fe2O3 + 3CO ⎯→ 2Fe + 3CO2
c)
2K + F2 ⎯→ 2KF
d)
CuO + H2 ⎯→ Cu + H2O
|
|
Raghav Bansal answered |
a) BaCl2 + H2SO4 → BaSO4 + 2HCl is not a redox reaction, as there is no change in the oxidation state of any element.
It is an example of double displacement reactions.
It is an example of double displacement reactions.
In the following reaction, 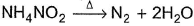 , oxidation number of
, oxidation number of- a)N changes from -2 to +2
- b)N changes from -2 to 0
- c)N in
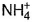 changes from -3 to 0 and that in
changes from -3 to 0 and that in 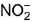 changes from +3 to 0
changes from +3 to 0 - d)N does not change
Correct answer is option 'C'. Can you explain this answer?
In the following reaction, 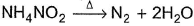 , oxidation number of
, oxidation number of
a)
N changes from -2 to +2
b)
N changes from -2 to 0
c)
N in 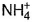 changes from -3 to 0 and that in
changes from -3 to 0 and that in 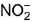 changes from +3 to 0
changes from +3 to 0
d)
N does not change

|
Learners Habitat answered |
In NH4NO2, the oxidation number of N in NH4+ is -3, and of N in NO2- is +3.
Oxidation numbers of P in PO4−3, of S in SO42− and that of Cr in Cr2O72− are respectively,- a) +5, +6 and +6
- b)+3, +6 and +5
- c)+5, +3 and +6
- d)-3, +6 and +6
Correct answer is option 'A'. Can you explain this answer?
Oxidation numbers of P in PO4−3, of S in SO42− and that of Cr in Cr2O72− are respectively,
a)
+5, +6 and +6
b)
+3, +6 and +5
c)
+5, +3 and +6
d)
-3, +6 and +6
|
|
Raghav Bansal answered |
The correct answer is option A
(I) xPO43− ⇒ x + 4 × (−2) = −3
⇒x = −3 + 8 = +5
⇒x = +5
Oxidation number of P = +5
(II) xSO42− ⇒ x + 4 × (−2) = −2
⇒x = −2 + 8
⇒x = +6
Oxidation number of S=+6
(III) xCr2O72− ⇒2x + 7 × (−2) = −2
⇒2x =−2+14
⇒2x=12
⇒x= 12/2 = +6
(I) xPO43− ⇒ x + 4 × (−2) = −3
⇒x = −3 + 8 = +5
⇒x = +5
Oxidation number of P = +5
(II) xSO42− ⇒ x + 4 × (−2) = −2
⇒x = −2 + 8
⇒x = +6
Oxidation number of S=+6
(III) xCr2O72− ⇒2x + 7 × (−2) = −2
⇒2x =−2+14
⇒2x=12
⇒x= 12/2 = +6
When an air bubble of radius R lies at a depth h below the free surface of a liquid of density ρ and surface tension Sla, then the excess pressure inside the bubble will be- a)
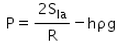
- b)
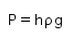
- c)
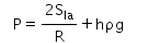
- d)
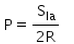
Correct answer is option 'C'. Can you explain this answer?
When an air bubble of radius R lies at a depth h below the free surface of a liquid of density ρ and surface tension Sla, then the excess pressure inside the bubble will be
a)
b)
c)
d)

|
Ambition Institute answered |
Excess pressure inside a cavity or air bubble in liquid formula
P[excess] = P[inside] - P [outside]
P [outside] =P[atm]
P[inside]= P[atm] +hpg [here p represents density] +2T/R
P[excess] = P[atm] +hpg [here p represents density] +2T/R-P[atm]
P[excess] = hpg+2T/R
Now substitute value for T
P[excess] = hpg+2S/R
Hence C is correct.
P[excess] = P[inside] - P [outside]
P [outside] =P[atm]
P[inside]= P[atm] +hpg [here p represents density] +2T/R
P[excess] = P[atm] +hpg [here p represents density] +2T/R-P[atm]
P[excess] = hpg+2T/R
Now substitute value for T
P[excess] = hpg+2S/R
Hence C is correct.
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Match the compounds/ions having underlined atoms of different oxidation number (in Column I) with values (in Column II).
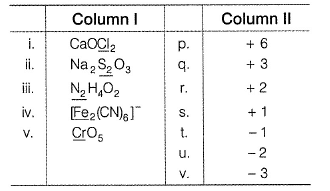
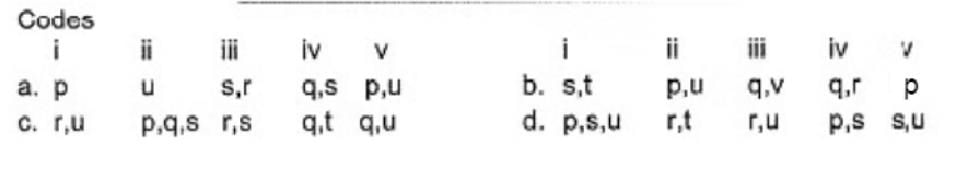

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Match the compounds/ions having underlined atoms of different oxidation number (in Column I) with values (in Column II).


a)
a
b)
b
c)
c
d)
d
|
|
Suresh Iyer answered |
(i) CaOCI2 has
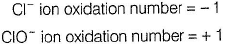
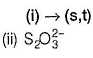
Oxidation number = + 6
Oxidation number = - 2
(ii) → (p,u)
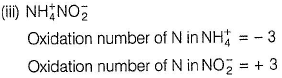
(iii) → (q.v)
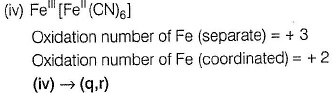
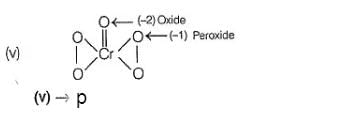
Oxidation number = + 6
Oxidation number = - 2
(ii) → (p,u)
(iii) → (q.v)

The angle of contact for liquid on a solid surface is the angle between:- a)the tangent to the liquid surface at the point of contact and the solid surface
- b)the tangent to the solid surface at the point of contact and the liquid surface
- c)the liquid surface and the solid surface at the point of contact
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
The angle of contact for liquid on a solid surface is the angle between:
a)
the tangent to the liquid surface at the point of contact and the solid surface
b)
the tangent to the solid surface at the point of contact and the liquid surface
c)
the liquid surface and the solid surface at the point of contact
d)
none of these
|
|
Raghav Bansal answered |
The angle of contact for liquid on a solid surface is the angle between the tangent to the liquid surface at the point of contact and the solid surface. This is the definition of angle of contact.
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion 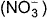 when freshly prepared FeSO4 solution is added to aqueous solution of
when freshly prepared FeSO4 solution is added to aqueous solution of 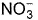 followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).Q. Magnetic moment
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).Q. Magnetic moment 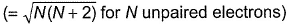 of Fe in the ring is
of Fe in the ring is - a)zero BM
- b)
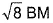
- c)
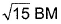
- d)
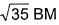
Correct answer is option 'C'. Can you explain this answer?
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion 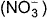 when freshly prepared FeSO4 solution is added to aqueous solution of
when freshly prepared FeSO4 solution is added to aqueous solution of 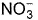 followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q. Magnetic moment 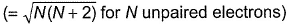 of Fe in the ring is
of Fe in the ring is
a)
zero BM
b)
c)
d)

|
Knowledge Hub answered |
Fe2+ is added asFeSO4
Fe+ is formed by charge transfer from NO to Fe2+
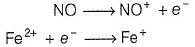

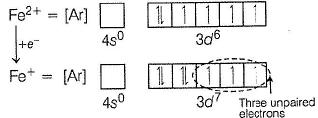
Fe+ has three unpaired electrons (N).
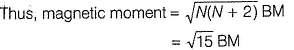
Fe+ is formed by charge transfer from NO to Fe2+
Fe+ has three unpaired electrons (N).
When impurity is added to a liquid, its surface tension- a)decreases
- b)first decreases and then increases
- c)increases
- d)remains same
Correct answer is option 'A'. Can you explain this answer?
When impurity is added to a liquid, its surface tension
a)
decreases
b)
first decreases and then increases
c)
increases
d)
remains same
|
|
Arun Khanna answered |
When impurities are mixed in liquid, surface tension of liquid decreases. The soluble substances when dissolved in water, decreases the surface tension of water.
When a capillary tube of radius r is dipped in a liquid of density ρ and surface tension S, the liquid rises or falls through a distance- a)
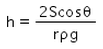
- b)
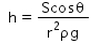
- c)
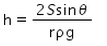
- d)
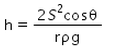
Correct answer is option 'A'. Can you explain this answer?
When a capillary tube of radius r is dipped in a liquid of density ρ and surface tension S, the liquid rises or falls through a distance
a)
b)
c)
d)

|
Ambition Institute answered |
As we know,
S=rhdg/2cosθ
⇒ Pressure difference =hdg=2Scosθ/r
Direction (Q. Nos. 13 and 14) This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.Q. Which of the following species has/have oxidation number of the metal as + 6 ?- a)
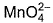
- b)
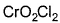
- c)
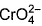
- d)

Correct answer is option 'A,B,C'. Can you explain this answer?
Direction (Q. Nos. 13 and 14) This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following species has/have oxidation number of the metal as + 6 ?
a)
b)
c)
d)

|
Knowledge Hub answered |
In which of the following reactions oxidation number of chromium has been affected?- a)
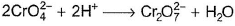
- b)
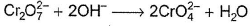
- c)
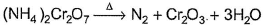
- d)

Correct answer is option 'C'. Can you explain this answer?
In which of the following reactions oxidation number of chromium has been affected?
a)
b)
c)
d)

|
Sankar Bose answered |
Oxidation number of Cr changes from +6 to +3.
Respiratory substrates are the organic substances which are ________ during respiration to liberate energy.- a)Oxidised
- b)Reduced
- c)Synthesised
- d)Both (a) and (b)
Correct answer is option 'A'. Can you explain this answer?
Respiratory substrates are the organic substances which are ________ during respiration to liberate energy.
a)
Oxidised
b)
Reduced
c)
Synthesised
d)
Both (a) and (b)
|
|
Dev Patel answered |
Respiration is an oxidative process in which repiratory substrates are oxidised to liberate energy inside the living cells. The common respiratory substrates are carbohydrates, proteins, fats and organic acids. The most common respiratory substrates are carbohydrates, proteins, fats and organic acids. The most common respiratory substrate is glucose.
RQ in anaerobic respiration is?- a)0.7
- b)0.9
- c)Unity
- d)Infinity
Correct answer is option 'D'. Can you explain this answer?
RQ in anaerobic respiration is?
a)
0.7
b)
0.9
c)
Unity
d)
Infinity
|
|
Suresh Iyer answered |
In anaerobic respiration, there is no consumption of oxygen and carbon dioxide is produced in most of the cases. Therefore, respiratory quotient is infinity. Carb the usual substarte.

Identify the step in tricarboxylic acid cycle, which does not involve oxidation of substrate?- a)
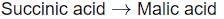
- b)
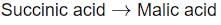
- c)
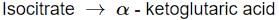
- d)
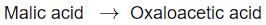
Correct answer is option 'B'. Can you explain this answer?
Identify the step in tricarboxylic acid cycle, which does not involve oxidation of substrate?
a)
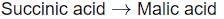
b)
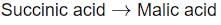
c)
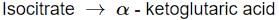
d)
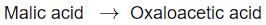
|
|
Sravya Sarkar answered |
Understanding the TCA Cycle Step
The tricarboxylic acid (TCA) cycle, also known as the Krebs cycle, is a crucial metabolic pathway involved in cellular respiration. Among its various steps, one specific reaction does not involve the oxidation of the substrate.
Step Analysis
In the context of the options provided, we analyze each step:
- a) Succinic acid → Malic Acid
- This step involves the oxidation of succinic acid to fumaric acid, followed by hydration to malic acid.
- b) Succinyl-CoA → Succinic acid
- This step is a substrate-level phosphorylation. Here, succinyl-CoA is converted to succinic acid, resulting in the production of GTP (or ATP). Importantly, there is no transfer of electrons or oxidation occurring in this step.
- c) Isocitrate → α-Ketoglutaric acid
- This reaction involves the oxidation of isocitrate, resulting in the production of α-ketoglutaric acid and the reduction of NAD+ to NADH.
- d) Malic acid → Oxaloacetic Acid
- In this step, malic acid is oxidized to oxaloacetic acid, generating NADH in the process.
Conclusion
The correct answer is option B: Succinyl-CoA → Succinic acid. This step is unique as it does not involve oxidation but rather a direct conversion facilitated by substrate-level phosphorylation, making it a key differentiator from the other steps in the TCA cycle.
The tricarboxylic acid (TCA) cycle, also known as the Krebs cycle, is a crucial metabolic pathway involved in cellular respiration. Among its various steps, one specific reaction does not involve the oxidation of the substrate.
Step Analysis
In the context of the options provided, we analyze each step:
- a) Succinic acid → Malic Acid
- This step involves the oxidation of succinic acid to fumaric acid, followed by hydration to malic acid.
- b) Succinyl-CoA → Succinic acid
- This step is a substrate-level phosphorylation. Here, succinyl-CoA is converted to succinic acid, resulting in the production of GTP (or ATP). Importantly, there is no transfer of electrons or oxidation occurring in this step.
- c) Isocitrate → α-Ketoglutaric acid
- This reaction involves the oxidation of isocitrate, resulting in the production of α-ketoglutaric acid and the reduction of NAD+ to NADH.
- d) Malic acid → Oxaloacetic Acid
- In this step, malic acid is oxidized to oxaloacetic acid, generating NADH in the process.
Conclusion
The correct answer is option B: Succinyl-CoA → Succinic acid. This step is unique as it does not involve oxidation but rather a direct conversion facilitated by substrate-level phosphorylation, making it a key differentiator from the other steps in the TCA cycle.
By which phenomenon does the water rise from roots to leaves of plants?- a)Capillary action
- b)Surface Tension
- c)Bernoulli’s Theorem
- d)Viscosity
Correct answer is option 'A'. Can you explain this answer?
By which phenomenon does the water rise from roots to leaves of plants?
a)
Capillary action
b)
Surface Tension
c)
Bernoulli’s Theorem
d)
Viscosity
|
|
Arun Khanna answered |
Plants use capillary action to bring water from the soil up through capillaries, small tubes in the plants, to the rest of the plant. Due to gravity, capillary action is not strong enough for the water to travel to the top of the plant. Another process, transpiration pull does the rest of the work.
the oxidation number of sulphur in S8,S2F2,H2S respectively are- a)0,+1 and -2
- b)+2, +1 and -2
- c)0, +1 and +2
- d)-2, +1 and -2
Correct answer is option 'A'. Can you explain this answer?
the oxidation number of sulphur in S8,S2F2,H2S respectively are
a)
0,+1 and -2
b)
+2, +1 and -2
c)
0, +1 and +2
d)
-2, +1 and -2
|
|
Sparsh Datta answered |
(i) Oxidation state of element in its free state is zero.
(ii) Sum of oxidation states of all atoms in compound is zero.
O.N of S in S8=0; O.N of S in S2F2=+1
O.N of S in H2S=-2
Which of the following atom has been assigned only single oxidation number?- a)H
- b)O
- c)N
- d)F
Correct answer is option 'D'. Can you explain this answer?
Which of the following atom has been assigned only single oxidation number?
a)
H
b)
O
c)
N
d)
F

|
Amrita Kumar answered |
Fluorine is the most electronegative element. It can gain one electron
F+ e- → F
-
Oxidation number = -1
Due to very high (IE), it cannot lose electron.
(a) HH- (-1), (H+ (+1)
(b) O -2 in oxide
-1 in peroxide
+2 in OF2
(c) N - 3 in N2O3, NH3
+3 in HNO2,
+5 in HNO3,
+ 1 in N2O
If drops and bubbles do not collapse under the effect of gravity, it indicates that- a)pressure inside the drop is greater than outside
- b)pressure inside the drop is lower than outside it
- c)Surface tension is low
- d)Viscosity is large
Correct answer is option 'A'. Can you explain this answer?
If drops and bubbles do not collapse under the effect of gravity, it indicates that
a)
pressure inside the drop is greater than outside
b)
pressure inside the drop is lower than outside it
c)
Surface tension is low
d)
Viscosity is large

|
Bhawna Mehta answered |
If drops and bubbles do not collapse under the effect of gravity, it indicates that the pressure inside the drop is greater than the pressure outside. The greater inner pressure prevents the drop from collapsing.
The value of respiratory Quotient actually depends upon- a)The type of respiratory substrate used
- b)The place in which respiration is occurring
- c)The amount of ventilation
- d)Breathing surface
Correct answer is option 'A'. Can you explain this answer?
The value of respiratory Quotient actually depends upon
a)
The type of respiratory substrate used
b)
The place in which respiration is occurring
c)
The amount of ventilation
d)
Breathing surface

|
Stepway Academy answered |
The correct answer is: (a) The type of respiratory substrate used
The Respiratory Quotient (RQ) is the ratio of the volume of carbon dioxide (CO₂) produced to the volume of oxygen (O₂) consumed during respiration. Its value depends primarily on the type of respiratory substrate being metabolized:
- Carbohydrates: RQ = 1 (equal amounts of O₂ consumed and CO₂ produced).
- Fats: RQ < 1 (more O₂ consumed than CO₂ produced).
- Proteins: RQ ≈ 0.8 (less O₂ consumed than CO₂ produced).
Other factors, such as location or ventilation, do not directly determine the RQ.
In which of the following set of compounds oxidation number of oxygen is not (- 2)?- a)KO2, OF2, H2O2
- b)
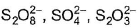
- c)CO2, CrO3, P2O3
- d)HNO2, HNO3, N2O
Correct answer is option 'A'. Can you explain this answer?
In which of the following set of compounds oxidation number of oxygen is not (- 2)?
a)
KO2, OF2, H2O2
b)
c)
CO2, CrO3, P2O3
d)
HNO2, HNO3, N2O

|
Anjana Desai answered |
OF2 Fluorine is most electronegative atom with oxidation number = - 1
x - 2 = 0
x = + 2
H2O2 2 + 2x = 0 ⇒ x = - 1
The excess pressure inside a soap bubble is (Here, Sla is the surface tension between the liquid-air interface).- a)
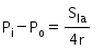
- b)
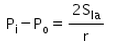
- c)
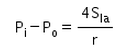
- d)
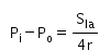
Correct answer is option 'C'. Can you explain this answer?
The excess pressure inside a soap bubble is (Here, Sla is the surface tension between the liquid-air interface).
a)
b)
c)
d)
|
|
Neha Joshi answered |
Excess pressure in water bubble = 2T/r (due to 1 surface only)
Excess pressure in a soap bubble = 4T/r (due to 2 surfaces)
Excess pressure in a soap bubble = 4T/r (due to 2 surfaces)
Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)Q. 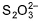 has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
Correct answer is '8'. Can you explain this answer?
Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. 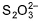 has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
|
|
Rohan Singh answered |
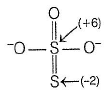
In ion S2O3(2-) , There are two sulphur atoms are present in the structure , as one sulphur atom is opposite to another. Sulphur atom has Oxidation state of +6, and another one is in the oxidation state of -2 as same as O atom in the structure.
The most common oxidation states of S are (-2,+4 and +6) the Ion S2O3(2-) which is S-SO3 is a combination of S(2-) and S(6+).
Refer the given equation.2(C51H98O6) + 145O2 → 102CO2 + 98H2O + EnergyThe RQ in this case is- a)1
- b)0.7
- c)1.45
- d)1.62
Correct answer is option 'B'. Can you explain this answer?
Refer the given equation.
2(C51H98O6) + 145O2 → 102CO2 + 98H2O + Energy
The RQ in this case is
a)
1
b)
0.7
c)
1.45
d)
1.62
|
|
Dev Patel answered |
When facts are used in respiration, the RQ is less than 1, e.g. for the breakdown of tripalmitin,
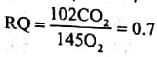
SI unit of surface tension is- a)N.m2
- b)N.m
- c)N/m
- d)N/m2
Correct answer is option 'C'. Can you explain this answer?
SI unit of surface tension is
a)
N.m2
b)
N.m
c)
N/m
d)
N/m2

|
Supriya Senapati answered |
Surface tension is defined as the ratio of surface force applied on a liquid to the length along which the force acts. so it is newton/meter
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion 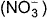 when freshly prepared FeSO4 solution is added to aqueous solution of
when freshly prepared FeSO4 solution is added to aqueous solution of 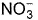 followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).Q.Oxidation number of the Fe in the ring is
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).Q.Oxidation number of the Fe in the ring is - a)+1
- b)+2
- c)+3
- d)+4
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion 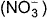 when freshly prepared FeSO4 solution is added to aqueous solution of
when freshly prepared FeSO4 solution is added to aqueous solution of 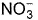 followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q.Oxidation number of the Fe in the ring is
a)
+1
b)
+2
c)
+3
d)
+4

|
Nandini Chakraborty answered |
Let, oxidation number of Fe = x
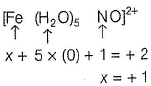
Chapter doubts & questions for November Week 1 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of November Week 1 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.