All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of July Week 4 for NEET Exam
Two thin long parallel wires separated by a distance b are carrying a current i ampere each. The magnitude of the force per unit length exerted by one wire on the other is- a)

- b)
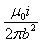
- c)
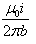
- d)
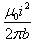
Correct answer is option 'D'. Can you explain this answer?
Two thin long parallel wires separated by a distance b are carrying a current i ampere each. The magnitude of the force per unit length exerted by one wire on the other is
a)
b)
c)
d)

|
EduRev Humanities answered |
Given, i1=i2=i
∴F=μ0i2l/2πb
Hence, force per unit length is F=μ0i2/2πb
∴F=μ0i2l/2πb
Hence, force per unit length is F=μ0i2/2πb
An electron and proton enter a magnetic field with equal velocities. Which one of them experiences a greater force?- a)electron
- b)proton
- c)Both experience same magnitude of force
- d)No prediction can be made.
Correct answer is option 'C'. Can you explain this answer?
An electron and proton enter a magnetic field with equal velocities. Which one of them experiences a greater force?
a)
electron
b)
proton
c)
Both experience same magnitude of force
d)
No prediction can be made.

|
Divey Sethi answered |
As charges and velocities are same
F=q(V×B)
So having the same magnitude of charge and same velocity, they'll experience the same magnitude of force.
F=q(V×B)
So having the same magnitude of charge and same velocity, they'll experience the same magnitude of force.
The correct statements among the given are
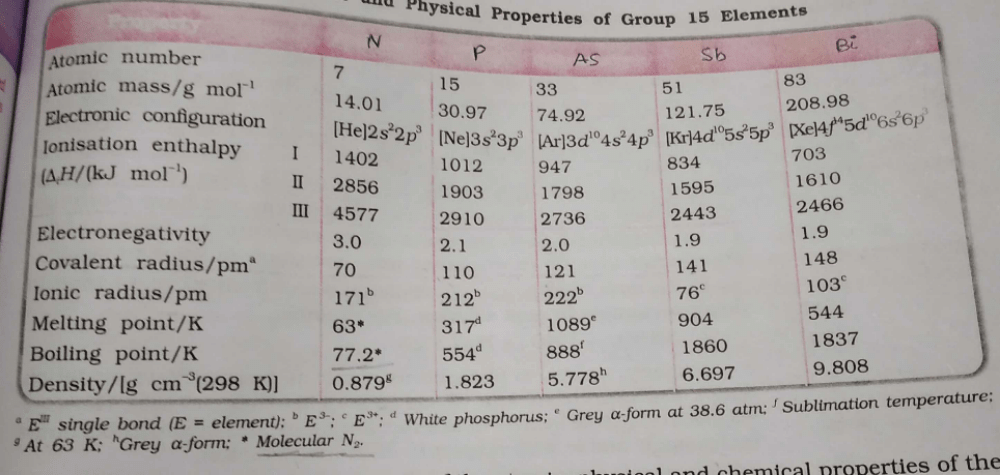
- a)Antimony belongs to 15th group and 5th period
- b)electron gain enthalpy of P > N > S > O
- c)Minimum and maximum oxidation number of phosphorus is -3 and +6
- d)Fluoroapatite, formula is Ca6(PO4)6 CaF2
Correct answer is option 'A'. Can you explain this answer?
The correct statements among the given are
a)
Antimony belongs to 15th group and 5th period
b)
electron gain enthalpy of P > N > S > O
c)
Minimum and maximum oxidation number of phosphorus is -3 and +6
d)
Fluoroapatite, formula is Ca6(PO4)6 CaF2

|
Divey Sethi answered |
Option A: Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi).
Option B: The electron gain enthalpy of P< N< S< O.
Option C: Minimum and maximum oxidation number of phosphorus are -3 and +5 respectively.
Option D: Fluorapatite is a phosphate mineral with the formula Ca5(PO4)3F .
Option B: The electron gain enthalpy of P< N< S< O.
Option C: Minimum and maximum oxidation number of phosphorus are -3 and +5 respectively.
Option D: Fluorapatite is a phosphate mineral with the formula Ca5(PO4)3F .
Hence, option A is correct.
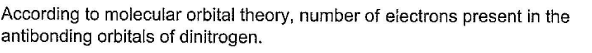
Correct answer is '4'. Can you explain this answer?
|
|
Subhankar Choudhary answered |
Molecular Orbital Theory and Antibonding Orbitals in Nitrogen
Molecular orbital theory (MOT) is a theoretical model that describes the behavior of electrons in molecules based on the principles of quantum mechanics. It is used to explain and predict the properties of molecules, including their electronic and magnetic properties, bond lengths, bond angles, and so on.
In MOT, the electrons in a molecule are treated as waves that are described by molecular orbitals (MOs), which are mathematical functions that represent the probability of finding an electron at a given point in space. These MOs are formed by combining the atomic orbitals of the atoms in the molecule.
Antibonding orbitals are MOs that have a higher energy than the atomic orbitals from which they are formed. When electrons occupy these orbitals, they weaken the bond between the atoms in the molecule, making it more likely to break apart.
Nitrogen has five valence electrons, which are represented by the atomic orbitals s and p. In the molecule N2, these atomic orbitals combine to form five MOs: two bonding MOs, two antibonding MOs, and one nonbonding MO.
The two bonding MOs are lower in energy than the atomic orbitals from which they are formed, and they help to hold the two nitrogen atoms together. The nonbonding MO is filled with two electrons, which are shared equally between the two nitrogen atoms and do not contribute to the bond strength.
The two antibonding MOs are higher in energy than the atomic orbitals from which they are formed, and they weaken the bond between the two nitrogen atoms. When all five valence electrons are placed into the MOs, there are four electrons in the antibonding MOs and one electron in the nonbonding MO.
Therefore, according to molecular orbital theory, there are four electrons present in the antibonding orbitals of nitrogen.
Molecular orbital theory (MOT) is a theoretical model that describes the behavior of electrons in molecules based on the principles of quantum mechanics. It is used to explain and predict the properties of molecules, including their electronic and magnetic properties, bond lengths, bond angles, and so on.
In MOT, the electrons in a molecule are treated as waves that are described by molecular orbitals (MOs), which are mathematical functions that represent the probability of finding an electron at a given point in space. These MOs are formed by combining the atomic orbitals of the atoms in the molecule.
Antibonding orbitals are MOs that have a higher energy than the atomic orbitals from which they are formed. When electrons occupy these orbitals, they weaken the bond between the atoms in the molecule, making it more likely to break apart.
Nitrogen has five valence electrons, which are represented by the atomic orbitals s and p. In the molecule N2, these atomic orbitals combine to form five MOs: two bonding MOs, two antibonding MOs, and one nonbonding MO.
The two bonding MOs are lower in energy than the atomic orbitals from which they are formed, and they help to hold the two nitrogen atoms together. The nonbonding MO is filled with two electrons, which are shared equally between the two nitrogen atoms and do not contribute to the bond strength.
The two antibonding MOs are higher in energy than the atomic orbitals from which they are formed, and they weaken the bond between the two nitrogen atoms. When all five valence electrons are placed into the MOs, there are four electrons in the antibonding MOs and one electron in the nonbonding MO.
Therefore, according to molecular orbital theory, there are four electrons present in the antibonding orbitals of nitrogen.
Hot conc. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and nonmetals. Which of the following element is oxidised by conc. H2SO4 into two gaseous products?- a) Cu
- b)S
- c)C
- d)zn
Correct answer is 'C'. Can you explain this answer?
Hot conc. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and nonmetals. Which of the following element is oxidised by conc. H2SO4 into two gaseous products?
a)
Cu
b)
S
c)
C
d)
zn
|
|
Naina Bansal answered |
C element is oxidised by conc. H2SO4 into two gaseous products.
In the third period of the periodic table the element having smallest size is - a)Na
- b)CI
- c)Ar
- d)Si
Correct answer is option 'B'. Can you explain this answer?
In the third period of the periodic table the element having smallest size is
a)
Na
b)
CI
c)
Ar
d)
Si
|
|
Aarav Sharma answered |
The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon.
In a period from left to right atomic size decreases due to Increase in nuclear charge.
but the noble gases are bigger than the halogens as they have octet and sort of repulsion occurs in the shells.
so the smallest element in a period is the halogen.so chlorine Cl is the smallest.
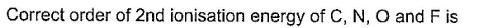
- a)
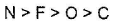
- b)
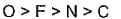
- c)
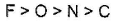
- d)
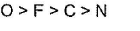
Correct answer is option 'B'. Can you explain this answer?
a)
b)
c)
d)
|
|
Nikita Singh answered |
The second ionization energy refers to the energy required to remove the electron from the corresponding monovalent cation of the respective atom.
It is expected to increase from left to right in the periodic table with the decrease in atomic size.
Since the Oxygen atom gets a stable electronic configuration, 2s22p3 after removing one electron, the O+ shows greater ionization energy than F+ as well as N+.
Thus, correct order will be: O > F > N > C
A 2 cm long copper wire having mass of 6 gm, dips in to two mercury pools to form a complete circuit. The wire is placed between the poles of a magnet which causes a field strength of 0.3 T. Find the initial upward acceleration of copper wire after 100 A of current is passed through the wire (g = 10 m/s2)- a)1 m/s2
- b)10 m/s2
- c)100 m/s2
- d)90 m/s2
Correct answer is option 'D'. Can you explain this answer?
A 2 cm long copper wire having mass of 6 gm, dips in to two mercury pools to form a complete circuit. The wire is placed between the poles of a magnet which causes a field strength of 0.3 T. Find the initial upward acceleration of copper wire after 100 A of current is passed through the wire (g = 10 m/s2)
a)
1 m/s2
b)
10 m/s2
c)
100 m/s2
d)
90 m/s2
|
|
Hansa Sharma answered |
ma=Bil−mg
a=0.3×100×2×10−2−10×6×10−3/ m
a= 0.6−0.06/6×10−3
=100−10
=90ms−2
a=0.3×100×2×10−2−10×6×10−3/ m
a= 0.6−0.06/6×10−3
=100−10
=90ms−2
A brown ring is formed in the ring test for NO3– ion. It is due to the formation of- a)[Fe(H2O)5 (NO)]2+
- b) FeSO4.NO2
- c)[Fe(H2O)4(NO)2]2+
- d)FeSO4.HNO3
Correct answer is option 'A'. Can you explain this answer?
A brown ring is formed in the ring test for NO3– ion. It is due to the formation of
a)
[Fe(H2O)5 (NO)]2+
b)
FeSO4.NO2
c)
[Fe(H2O)4(NO)2]2+
d)
FeSO4.HNO3

|
Sushil Kumar answered |
When freshly prepared solution of FeSO4 is added in a solution containing NO3– ion, it leads to formation of a brown coloured complex. This is known as brown ring test of nitrate.
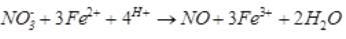
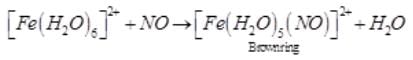
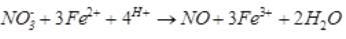
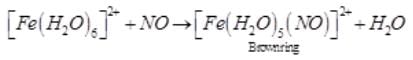
- a)
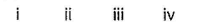

- b)
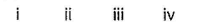

- c)
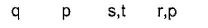
- d)
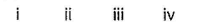
Correct answer is option 'A'. Can you explain this answer?
a)
b)
c)
d)
|
|
Priyanka Sharma answered |
(i) Nitrogen is a non-metal.
(ii) Phosphorus is a non-metal.
(iii) Arsenic is a metalloid and shows Sublimation.
(iv) Bismuth is metal and shows the Inert pair effect.
(ii) Phosphorus is a non-metal.
(iii) Arsenic is a metalloid and shows Sublimation.
(iv) Bismuth is metal and shows the Inert pair effect.
Hence, option A is correct.
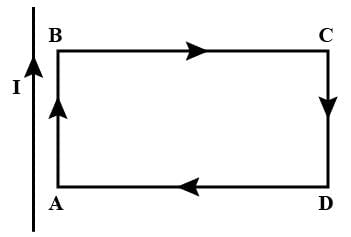
A rectangular loop carrying a current I is situated near a long straight wire such that the wire is parallel to the one of the sides of the loop and is in a plane of the loop. If a steady current I is established in wire as shown in figure, the loop will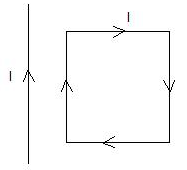
- a)move away from the wire or towards right
- b)remain stationary
- c)rotate about an axis parallel to the wire
- d)move towards the wire
Correct answer is option 'D'. Can you explain this answer?
A rectangular loop carrying a current I is situated near a long straight wire such that the wire is parallel to the one of the sides of the loop and is in a plane of the loop. If a steady current I is established in wire as shown in figure, the loop will
a)
move away from the wire or towards right
b)
remain stationary
c)
rotate about an axis parallel to the wire
d)
move towards the wire

|
Top Rankers answered |
The long straight wire and side AB carry current in the same direction, hence will attract each other.
The long straight wire and side CD carry current in the opposite direction, hence will repel each other.
Force on side BC will be equal and opposite to force on side DA.
Since CD is farther from the wire than AB, the force of attraction on AB will exceed the force of repulsion on CD.
Hence, there will be a net force of attraction on the loop ABCD and it will move towards the wire.
The long straight wire and side CD carry current in the opposite direction, hence will repel each other.
Force on side BC will be equal and opposite to force on side DA.
Since CD is farther from the wire than AB, the force of attraction on AB will exceed the force of repulsion on CD.
Hence, there will be a net force of attraction on the loop ABCD and it will move towards the wire.

- a)
- b)

- c)

- d)
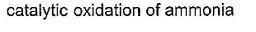
Correct answer is option 'B'. Can you explain this answer?
a)
b)
c)
d)
|
|
Gauri Datta answered |
Oxidation of ammonia with CuO produces nitrogen gas and water vapor. This reaction is represented as:
2NH3 + 3CuO → 3Cu + N2 + 3H2O
The gaseous chemical produced in this reaction is nitrogen gas (N2), which is also obtained by reacting excess ammonia with chlorine. This reaction is represented as:
2NH3 + Cl2 → N2 + 2HCl
Explanation:
- Ammonium nitrate: Heating ammonium nitrate results in the decomposition of ammonium nitrate into nitrogen gas, water vapor, and oxygen gas. The reaction is represented as:
NH4NO3 → N2 + 2H2O + O2
- Potassium dichromate: Heating potassium dichromate results in the production of oxygen gas and potassium chromate. The reaction is represented as:
4K2Cr2O7 → 4K2CrO4 + 3O2
- Catalytic oxidation of ammonia: Catalytic oxidation of ammonia involves the use of a catalyst (such as platinum or palladium) to oxidize ammonia to nitrogen gas and water vapor. The reaction is represented as:
4NH3 + 5O2 → 4NO + 6H2O
2NO + O2 → 2NO2
4NO2 + O2 → 2N2O5
N2O5 → N2 + 2.5O2
- Reacting excess ammonia with chlorine: This reaction involves the reaction of excess ammonia with chlorine gas to produce nitrogen gas and hydrochloric acid. The reaction is represented as:
2NH3 + Cl2 → N2 + 2HCl
Therefore, option B, reacting excess ammonia with chlorine, is the correct answer.
2NH3 + 3CuO → 3Cu + N2 + 3H2O
The gaseous chemical produced in this reaction is nitrogen gas (N2), which is also obtained by reacting excess ammonia with chlorine. This reaction is represented as:
2NH3 + Cl2 → N2 + 2HCl
Explanation:
- Ammonium nitrate: Heating ammonium nitrate results in the decomposition of ammonium nitrate into nitrogen gas, water vapor, and oxygen gas. The reaction is represented as:
NH4NO3 → N2 + 2H2O + O2
- Potassium dichromate: Heating potassium dichromate results in the production of oxygen gas and potassium chromate. The reaction is represented as:
4K2Cr2O7 → 4K2CrO4 + 3O2
- Catalytic oxidation of ammonia: Catalytic oxidation of ammonia involves the use of a catalyst (such as platinum or palladium) to oxidize ammonia to nitrogen gas and water vapor. The reaction is represented as:
4NH3 + 5O2 → 4NO + 6H2O
2NO + O2 → 2NO2
4NO2 + O2 → 2N2O5
N2O5 → N2 + 2.5O2
- Reacting excess ammonia with chlorine: This reaction involves the reaction of excess ammonia with chlorine gas to produce nitrogen gas and hydrochloric acid. The reaction is represented as:
2NH3 + Cl2 → N2 + 2HCl
Therefore, option B, reacting excess ammonia with chlorine, is the correct answer.
Along an infinitely long conductor carrying a current of 8 A we keep another conductor of length 5 m carrying a current of 3 A. Both the conductors are 10 cm apart. Find the force on small conductor.- a)2.4 X 10-4 N
- b)9.6 X 10-4 N
- c)2.6 X 10-6 N
- d)9.6 X 10-5 N
Correct answer is option 'A'. Can you explain this answer?
Along an infinitely long conductor carrying a current of 8 A we keep another conductor of length 5 m carrying a current of 3 A. Both the conductors are 10 cm apart. Find the force on small conductor.
a)
2.4 X 10-4 N
b)
9.6 X 10-4 N
c)
2.6 X 10-6 N
d)
9.6 X 10-5 N
|
|
Shreya Singh answered |
F=4π×10^-7 i1 i2 l/2π rF= 4π × 10^-7×8×3× 5./2π×10^-2.F= 2×10^-7×8×3× 5 ×100.F=2.4×10^-4.
Which of the following molecular species has unpaired electron(s) ?- a)N2
- b)O2
- c)NO+
- d)CN-
Correct answer is option 'B'. Can you explain this answer?
Which of the following molecular species has unpaired electron(s) ?
a)
N2
b)
O2
c)
NO+
d)
CN-
|
|
Priya Chavan answered |
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
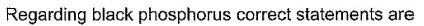
- a)

- b)
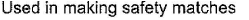
- c)
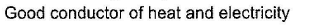
- d)
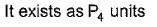
Correct answer is 'A,C'. Can you explain this answer?
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
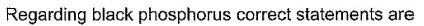
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
a)
b)
c)
d)

|
Rahul Desai answered |
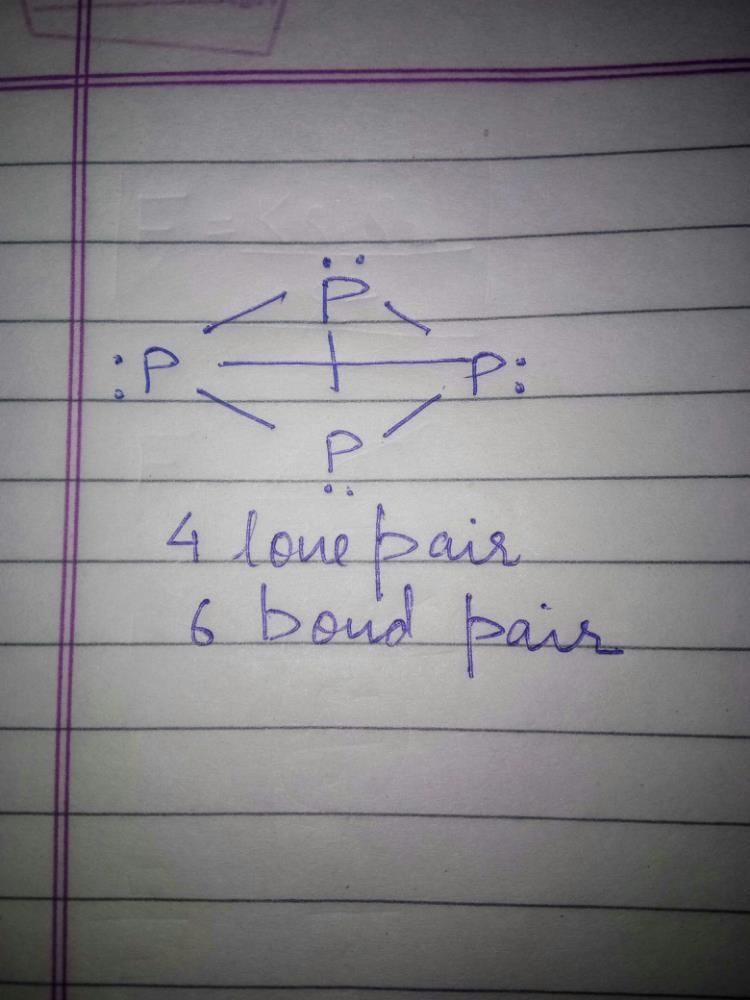
(a.c) Being goad conductor of neat and elecnicky, black phosphorus is a most stabte allotrope of phosphorus
Statement Type
This section is based on Statement I and Statement II. Select the correct anser from the codes given below

- a)

- b)

- c)
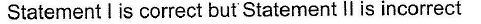
- d)
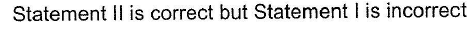
Correct answer is option 'C'. Can you explain this answer?
Statement Type
This section is based on Statement I and Statement II. Select the correct anser from the codes given below

This section is based on Statement I and Statement II. Select the correct anser from the codes given below
a)
b)
c)
d)

|
Pranjal Pillai answered |
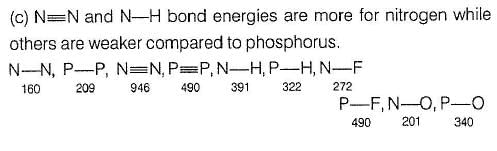
P — P single bond in P4 molecule is much weaker
(213 kJ mol-1) than N ≡ N triple bond (941.4 kJ mol-1) in N2.
The constant μo is called- a)Absolute Permeability
- b)Coefficient of mutual induction
- c)Coefficient of self induction
- d)Relative permeability
Correct answer is option 'A'. Can you explain this answer?
The constant μ
o
is calleda)
Absolute Permeability
b)
Coefficient of mutual induction
c)
Coefficient of self induction
d)
Relative permeability
|
|
Harsh Singhal answered |
B&c direct wrong
now for d relative permeability is Ur/U0
now for d relative permeability is Ur/U0
Number of chemical species having negative oxidation state for nitrogen among NF3, NCI3, NH2OH, NH3,CH3NH2, NH-2, L13N, N20, HCN, HNC, NO-2
Correct answer is '8'. Can you explain this answer?
Number of chemical species having negative oxidation state for nitrogen among
NF3, NCI3, NH2OH, NH3,CH3NH2, NH-2, L13N, N20, HCN, HNC, NO-2

|
Keerthana Mehta answered |
(8) Except in 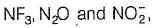 in all others N has negative oxidation states
in all others N has negative oxidation states
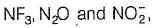 in all others N has negative oxidation states
in all others N has negative oxidation statesThe connecting wires of a battery of an automobile carry 200 A of current. Calculate the force per unit length between the wires if they are 50 cm long and 2 cm apart?- a)4Nm-1
- b)0.4Nm-1
- c)0.04Nm-1
- d)40Nm-1
Correct answer is option 'B'. Can you explain this answer?
The connecting wires of a battery of an automobile carry 200 A of current. Calculate the force per unit length between the wires if they are 50 cm long and 2 cm apart?
a)
4Nm-1
b)
0.4Nm-1
c)
0.04Nm-1
d)
40Nm-1
|
|
Keerthana Chakraborty answered |
Given parameters:
Current, I = 200 A
Length of the wire, l = 50 cm = 0.5 m
Distance between the wires, d = 2 cm = 0.02 m
To find: Force per unit length between the wires
Formula used: Magnetic force per unit length between two parallel conductors is given by the formula:
F/L = μ0 I1 I2 / 2πd
Where
F/L = force per unit length
μ0 = permeability of free space = 4π x 10^-7 Tm/A
I1, I2 = current in the two wires
d = distance between the wires
Substituting the given values in the above formula, we get:
F/L = (4π x 10^-7 Tm/A) x (200 A)^2 / 2π x 0.02 m
F/L = 0.4 N/m
Therefore, the force per unit length between the wires is 0.4 N/m, which is option (b).
Current, I = 200 A
Length of the wire, l = 50 cm = 0.5 m
Distance between the wires, d = 2 cm = 0.02 m
To find: Force per unit length between the wires
Formula used: Magnetic force per unit length between two parallel conductors is given by the formula:
F/L = μ0 I1 I2 / 2πd
Where
F/L = force per unit length
μ0 = permeability of free space = 4π x 10^-7 Tm/A
I1, I2 = current in the two wires
d = distance between the wires
Substituting the given values in the above formula, we get:
F/L = (4π x 10^-7 Tm/A) x (200 A)^2 / 2π x 0.02 m
F/L = 0.4 N/m
Therefore, the force per unit length between the wires is 0.4 N/m, which is option (b).
In two current carrying conductors parallel currents________, anti parallel currents_________ .- a)attract , attract
- b)attract , repel
- c)repel , attract
- d)repel , repel
Correct answer is option 'B'. Can you explain this answer?
In two current carrying conductors parallel currents________, anti parallel currents_________ .
a)
attract , attract
b)
attract , repel
c)
repel , attract
d)
repel , repel

|
Karan Sd answered |
In parallel wires the current are flowing in the same direction so they attract each other . if the current are flowing in opposite directions they repel each other
Which are correct statements ?
- a)The melting point of antimony is higher than bismuth
- b)Ionisation energy of C < O < N
- c)In 15th group, all show allotropy except bismuth and nitrogen
- d)Maximum covalency of nitrogen and Phosphorus are 4 and 5 respectively
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which are correct statements ?
a)
The melting point of antimony is higher than bismuth
b)
Ionisation energy of C < O < N
c)
In 15th group, all show allotropy except bismuth and nitrogen
d)
Maximum covalency of nitrogen and Phosphorus are 4 and 5 respectively
|
|
Om Desai answered |
Option A: Bismuth has 6 electron shells, whereas Antimony has 5 electron shells. Because of this, the attractive force between two Bismuth atoms is less due to electron shielding, resulting in bismuth possessing a lower boiling point than antimony.
Option B: In a period of moving from left to right, the ionization energy increases. Since N has a table half-filled 2p subshell which requires large energy.
Thus, the correct order of ionization energy is C < O < N.
Option C: Except N and Bi, all the group 15 elements exhibits allotropy. The allotropes of phosphorous are rather complex but essentially, there are three allotropic forms known as white, red, and black phosphorous.
Option D: Maximum covalency of N & P are 4 and 5.
Option B: In a period of moving from left to right, the ionization energy increases. Since N has a table half-filled 2p subshell which requires large energy.
Thus, the correct order of ionization energy is C < O < N.
Option C: Except N and Bi, all the group 15 elements exhibits allotropy. The allotropes of phosphorous are rather complex but essentially, there are three allotropic forms known as white, red, and black phosphorous.
Option D: Maximum covalency of N & P are 4 and 5.
Hence, option A,B,C,D is correct.
Which is the correct order w.r.t the given property? - a)N > P > As > Sb > Bi (Atomic mass)
- b)N > P > As > Sb = Bi (Electronegativity)
- c)N > P > As > Sb = Bi (Covalent radii)
- d)N = P > As > Sb > Bi (Density)
Correct answer is option 'B'. Can you explain this answer?
Which is the correct order w.r.t the given property?
a)
N > P > As > Sb > Bi (Atomic mass)
b)
N > P > As > Sb = Bi (Electronegativity)
c)
N > P > As > Sb = Bi (Covalent radii)
d)
N = P > As > Sb > Bi (Density)
|
|
Roshni Desai answered |
I'm sorry, but the question is incomplete. Please provide more information about the property being referred to.
Choose the correct answer from the alternatives given:
Watson and Crick (1953) proposed DNA double helix model and won the Nobel Prize; their model of DNA was based on
(i) X-ray diffraction studies of DNA done by Wilkins and Franklin.
(ii) Chargaffs base equivalence rule.
(iii) Griffiths transformation experiment.
(iv) Meselson and Stahls experiment.- a)(i), (ii) and (iv)
- b)(i) and (ii)
- c)(iii) and (iv)
- d)(i), (ii), (iii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
Choose the correct answer from the alternatives given:
Watson and Crick (1953) proposed DNA double helix model and won the Nobel Prize; their model of DNA was based on
(i) X-ray diffraction studies of DNA done by Wilkins and Franklin.
(ii) Chargaffs base equivalence rule.
(iii) Griffiths transformation experiment.
(iv) Meselson and Stahls experiment.
Watson and Crick (1953) proposed DNA double helix model and won the Nobel Prize; their model of DNA was based on
(i) X-ray diffraction studies of DNA done by Wilkins and Franklin.
(ii) Chargaffs base equivalence rule.
(iii) Griffiths transformation experiment.
(iv) Meselson and Stahls experiment.
a)
(i), (ii) and (iv)
b)
(i) and (ii)
c)
(iii) and (iv)
d)
(i), (ii), (iii) and (iv)
|
|
Anjali Sharma answered |
In 1953, James Watson and Francis Crick based on the X-ray diffraction data produced by Maurice Wilkins and Rosalind Franklin, proposed double helix model for the structure of DNA. One of the hallmarks of their proposition was base pairing between two strands of polynucleotide chains. However, this proportion was also based on the observation of Erwin Chargaff (1950) that for a double stranded DNA, the ratios between Adenine and Thymine and Guanine and Cytosine are constant and equals one.
Which of the following phenomena was experimentally proved by Meselson and Stahl?- a)Transformation
- b)Transduction
- c)Semi-conservative DNA replication
- d)Central dogma
Correct answer is option 'C'. Can you explain this answer?
Which of the following phenomena was experimentally proved by Meselson and Stahl?
a)
Transformation
b)
Transduction
c)
Semi-conservative DNA replication
d)
Central dogma
|
|
Anjali Sharma answered |
The Meselson and Stahl experiment was an experiment to prove that DNA replication was semi-subsequently and it was first shown in Escherichia coli and subsequently in higher organisms, such as plants and human cells. Semi-conservative replication means that when the double stranded DNA helix was replicated, each pf the two double stranded DNA helices consisted of one strand coming from the parental helix and one is newly synthesised.
To prove that DNA is the genetic material, which radioactive isotopes were used by Hershey and Chase(1952) in their experiments?- a)35S and 15N
- b)32P and 35S
- c)32P and 15N
- d)14N and 5N
Correct answer is option 'B'. Can you explain this answer?
To prove that DNA is the genetic material, which radioactive isotopes were used by Hershey and Chase(1952) in their experiments?
a)
35S and 15N
b)
32P and 35S
c)
32P and 15N
d)
14N and 5N
|
|
Vivek Patel answered |
Hershey and Chase experiment is based on the fact that DNA contains phosphorus and similarly, sulphur is present in proteins but not in DNA. They incorporated radioactive isotope of phosphorus (32P) into phage DNA and that of sulphur (35S) into proteins of a separate phage culture. Viruses grown in the presence of radioactive phosphorus contained radioactive DNA but not radioactive protein because DNA contains phosphorus but proteins do not, Similarly, viruses grown on radioactive sulphur contain radioactive- protein but not-radioactive DNA because DNA does not contain sulphur.
Q. The process of transformation is not affected by which of the following enzymes?
A. DNase
B. RNase
C. Peptidase
D. Lipase- a)A, B
- b)A, B, C, D
- c)B, C, D
- d)A, B, C
Correct answer is option 'C'. Can you explain this answer?
Q. The process of transformation is not affected by which of the following enzymes?
A. DNase
B. RNase
C. Peptidase
D. Lipase
A. DNase
B. RNase
C. Peptidase
D. Lipase
a)
A, B
b)
A, B, C, D
c)
B, C, D
d)
A, B, C
|
|
Gayatri Bose answered |
Answer:
The process of transformation is a genetic process in which foreign genetic material is introduced into a cell, resulting in a genetic change. In this process, the foreign DNA is taken up by the recipient cell and incorporated into its own genome. However, not all enzymes have an effect on the process of transformation.
Enzymes that do not affect the process of transformation:
The correct answer is option C, which includes the enzymes B. RNase, C. Peptidase, and D. Lipase. Let's understand why these enzymes do not have an effect on the process of transformation:
1. RNase:
RNase is an enzyme that degrades RNA molecules. However, in the process of transformation, the focus is on DNA molecules and their uptake by the recipient cell. Since RNase specifically targets RNA and not DNA, it does not have any effect on the transformation process.
2. Peptidase:
Peptidase is an enzyme that catalyzes the breakdown of peptides into amino acids. It is involved in protein metabolism and does not directly interact with DNA or RNA. Therefore, it does not affect the process of transformation.
3. Lipase:
Lipase is an enzyme that catalyzes the breakdown of lipids (fats). It does not have any direct involvement in DNA or RNA metabolism. Therefore, lipase has no effect on the process of transformation.
Enzymes that do affect the process of transformation:
The enzyme A. DNase, on the other hand, does have an effect on the process of transformation. DNase is an enzyme that degrades DNA molecules. If DNase is present in the environment during the transformation process, it can degrade the foreign DNA that has been taken up by the recipient cell, preventing its incorporation into the genome. Therefore, DNase can hinder the success of transformation.
To summarize, enzymes like DNase can have a negative impact on the process of transformation by degrading foreign DNA. However, enzymes like RNase, peptidase, and lipase do not affect the transformation process as they do not directly interact with DNA molecules. Therefore, the correct answer is option C.
The process of transformation is a genetic process in which foreign genetic material is introduced into a cell, resulting in a genetic change. In this process, the foreign DNA is taken up by the recipient cell and incorporated into its own genome. However, not all enzymes have an effect on the process of transformation.
Enzymes that do not affect the process of transformation:
The correct answer is option C, which includes the enzymes B. RNase, C. Peptidase, and D. Lipase. Let's understand why these enzymes do not have an effect on the process of transformation:
1. RNase:
RNase is an enzyme that degrades RNA molecules. However, in the process of transformation, the focus is on DNA molecules and their uptake by the recipient cell. Since RNase specifically targets RNA and not DNA, it does not have any effect on the transformation process.
2. Peptidase:
Peptidase is an enzyme that catalyzes the breakdown of peptides into amino acids. It is involved in protein metabolism and does not directly interact with DNA or RNA. Therefore, it does not affect the process of transformation.
3. Lipase:
Lipase is an enzyme that catalyzes the breakdown of lipids (fats). It does not have any direct involvement in DNA or RNA metabolism. Therefore, lipase has no effect on the process of transformation.
Enzymes that do affect the process of transformation:
The enzyme A. DNase, on the other hand, does have an effect on the process of transformation. DNase is an enzyme that degrades DNA molecules. If DNase is present in the environment during the transformation process, it can degrade the foreign DNA that has been taken up by the recipient cell, preventing its incorporation into the genome. Therefore, DNase can hinder the success of transformation.
To summarize, enzymes like DNase can have a negative impact on the process of transformation by degrading foreign DNA. However, enzymes like RNase, peptidase, and lipase do not affect the transformation process as they do not directly interact with DNA molecules. Therefore, the correct answer is option C.
Convert the following sentences into simple past passive.
They never sent me the bill.Correct answer is 'I was never sent the bill'. Can you explain this answer?
Convert the following sentences into simple past passive.
They never sent me the bill.
They never sent me the bill.
|
|
Disha Saha answered |
When we convert an active sentence in the simple past tense into the passive voice, we use the verb 'was/were + past participle'. 'Was' is used when the subject is a singular noun or pronoun.
So the answer is, 'I was never sent the bill'.
So the answer is, 'I was never sent the bill'.
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)PassagePhosphorus was discovered by Brand (1669), Scheele isolated from bone ash and Lavoisier proved its elemental nature (1777). The principal minerals are phosphate rock, fluoroapatite and chloroapatite. Phosphorus is prepared by the direct reduction of phosphorite by carbon in the presence of silica. It exists in different allotropic forms such as yellow or white, red, a-black,f3-black, etc. White P is most reactive, poisonous, glows in dark and readily catches fire due to unstable discrete P4 molecules. Red P is inert, non-poisonous, does not glow etc., due to its polymeric structure. a-black, f3 -black allotropes are also chemically inert, do not ignite at normal temperature. It has layer structure like graphite and acts as conductor.
Q.which of the following statements is/are correct ?- a)Abundance in the earth’s crust P>N>As>Sb
- b)N2 molecule has pπ - pπ bonding
- c)Among the nitrogen halides, NF3 is least stable
- d)None of the above
Correct answer is option 'A,B'. Can you explain this answer?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
Phosphorus was discovered by Brand (1669), Scheele isolated from bone ash and Lavoisier proved its elemental nature (1777). The principal minerals are phosphate rock, fluoroapatite and chloroapatite. Phosphorus is prepared by the direct reduction of phosphorite by carbon in the presence of silica. It exists in different allotropic forms such as yellow or white, red, a-black,f3-black, etc. White P is most reactive, poisonous, glows in dark and readily catches fire due to unstable discrete P4 molecules. Red P is inert, non-poisonous, does not glow etc., due to its polymeric structure. a-black, f3 -black allotropes are also chemically inert, do not ignite at normal temperature. It has layer structure like graphite and acts as conductor.
Q.
which of the following statements is/are correct ?
a)
Abundance in the earth’s crust P>N>As>Sb
b)
N2 molecule has pπ - pπ bonding
c)
Among the nitrogen halides, NF3 is least stable
d)
None of the above
|
|
Rishika Patel answered |
A molecule of N2 has the pπ-pπ bonding with each other respectively and the abundance in the earth's crest follows
P > N > As > Sb
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correctThe allotrope of phosphorus that has layer lattice like graphite is - a)white P
- b)yellow P
- c)red P
- d)black P
Correct answer is option 'D'. Can you explain this answer?
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
The allotrope of phosphorus that has layer lattice like graphite is
a)
white P
b)
yellow P
c)
red P
d)
black P

|
Piyush Jangir answered |
Black P has graphite layer like structure...

- a)
- b)
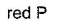
- c)
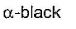
- d)
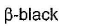
Correct answer is option 'A'. Can you explain this answer?
a)
b)
c)
d)

|
Nishtha Bose answered |
White P is an allotrope of phosphorus with low ignition temperature.
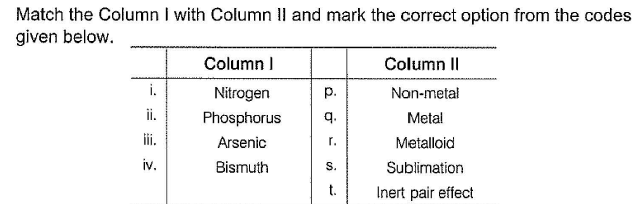
Match the Column I with Column II and mark the correct option from the codes given below :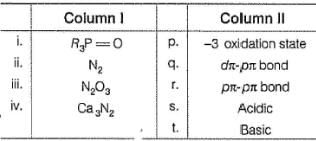
- a)

- b)
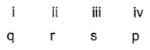
- c)
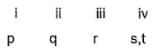
- d)

Correct answer is option 'B'. Can you explain this answer?
Match the Column I with Column II and mark the correct option from the codes given below :

a)

b)
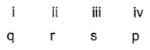
c)
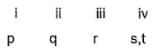
d)


|
Nisha Banerjee answered |
(i) → (q),(ii) → (r), (iii) → (s), (iv) → (p)
Choose the correct answer from the alternatives given:
Which of the following criteria should be fulfilled by a molecule to act as a genetic material?
(i) It should be able to replicate.
(ii) It should be structurally and chemically stable.
(iii) It should be able to undergo slow mutations.
(iv) It should be able to express itself in the form of Mendelian characters.- a)(i) and (ii)
- b)(ii) and (iii)
- c)(i), (ii) and (iii)
- d)(i), (ii), (iii) and (iv)
Correct answer is option 'D'. Can you explain this answer?
Choose the correct answer from the alternatives given:
Which of the following criteria should be fulfilled by a molecule to act as a genetic material?
(i) It should be able to replicate.
(ii) It should be structurally and chemically stable.
(iii) It should be able to undergo slow mutations.
(iv) It should be able to express itself in the form of Mendelian characters.
Which of the following criteria should be fulfilled by a molecule to act as a genetic material?
(i) It should be able to replicate.
(ii) It should be structurally and chemically stable.
(iii) It should be able to undergo slow mutations.
(iv) It should be able to express itself in the form of Mendelian characters.
a)
(i) and (ii)
b)
(ii) and (iii)
c)
(i), (ii) and (iii)
d)
(i), (ii), (iii) and (iv)
|
|
Jaideep Chauhan answered |
Criteria for a molecule to act as a genetic material
A molecule that acts as genetic material must fulfill certain criteria to ensure the proper transmission of genetic information from one generation to the next. The criteria are:
1. It should be able to replicate:
The molecule must be able to replicate itself accurately and efficiently so that the genetic information can be passed on to the next generation without any errors.
2. It should be structurally and chemically stable:
The molecule must be chemically and structurally stable so that it can withstand the various chemical and physical processes that occur during replication and cell division.
3. It should be able to undergo slow mutations:
The molecule must be able to undergo slow mutations, which are necessary for the generation of genetic diversity and evolution.
4. It should be able to express itself in the form of Mendelian characters:
The molecule must be able to express itself in the form of Mendelian characters, which are the observable traits that are inherited from one generation to the next.
Correct answer:
The correct answer is option D, which includes all the above criteria. A molecule that acts as genetic material must be able to replicate accurately, be structurally and chemically stable, undergo slow mutations, and express itself in the form of Mendelian characters.
A molecule that acts as genetic material must fulfill certain criteria to ensure the proper transmission of genetic information from one generation to the next. The criteria are:
1. It should be able to replicate:
The molecule must be able to replicate itself accurately and efficiently so that the genetic information can be passed on to the next generation without any errors.
2. It should be structurally and chemically stable:
The molecule must be chemically and structurally stable so that it can withstand the various chemical and physical processes that occur during replication and cell division.
3. It should be able to undergo slow mutations:
The molecule must be able to undergo slow mutations, which are necessary for the generation of genetic diversity and evolution.
4. It should be able to express itself in the form of Mendelian characters:
The molecule must be able to express itself in the form of Mendelian characters, which are the observable traits that are inherited from one generation to the next.
Correct answer:
The correct answer is option D, which includes all the above criteria. A molecule that acts as genetic material must be able to replicate accurately, be structurally and chemically stable, undergo slow mutations, and express itself in the form of Mendelian characters.
Chapter doubts & questions for July Week 4 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of July Week 4 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup