All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of January Week 1 for NEET Exam
Light of wavelength 500 nm is incident on a slit o... moref width 0.1 mm. The width of the central bright line on the screen is 2m. What is the distance of the screen?a)1 mb)200 mc)0.75 md)0.5 mCorrect answer is option 'B'. Can you explain this answer?
|
|
Swara Sharma answered |
Beta(central Maxima)=2lambda D(distance of the screen)/d(distance between slits).
beta=2m.
wavelength= 500nm=5×10^-7m.
d=0.1mm=1×10^-4m.
2=2×5×10^-7 D/10^-4.
1=5×10^-3 D .
1/5×10^-3=D .
1000/5=D.
200m=D
beta=2m.
wavelength= 500nm=5×10^-7m.
d=0.1mm=1×10^-4m.
2=2×5×10^-7 D/10^-4.
1=5×10^-3 D .
1/5×10^-3=D .
1000/5=D.
200m=D
Which of the following is not applicable for a biopatent?- a)Formation of new products
- b)Unearthing the DNA sequences
- c)Discovery of cell lines
- d)Using the existing technology to produce new products
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not applicable for a biopatent?
a)
Formation of new products
b)
Unearthing the DNA sequences
c)
Discovery of cell lines
d)
Using the existing technology to produce new products
|
|
Geetika Shah answered |
A biological patent is a patent on an invention in the field of biology that by law allows the patent holder to exclude others from making, using, selling, or importing the protected invention for a limited period of time. Hence, the correct option is c.
Diffraction of light gives the information of- a)Transverse nature
- b)Longitudinal nature
- c)Both transverse and longitudinal
- d)Neither transverse nor longitudina
Correct answer is option 'D'. Can you explain this answer?
Diffraction of light gives the information of
a)
Transverse nature
b)
Longitudinal nature
c)
Both transverse and longitudinal
d)
Neither transverse nor longitudina
|
|
Vijay Bansal answered |
Transverse and longitudinal waves. Light and other types of electromagnetic radiation are transverse waves. Water waves and S waves (a type of seismic wave) are also transverse waves. In transverse waves, the vibrations are at right angles to the direction of travel.
Diffraction pattern cannot be observed with:a)one wide slitb)two narrow slitsc)one narrow slitd)large number of narrow slitsCorrect answer is option 'A'. Can you explain this answer?
|
|
Pooja Mehta answered |
If one illuminates two parallel slits, the light from the two slits again interferes. Here the interference is a more pronounced pattern with a series of alternating light and dark bands. ... He also proposed (as a thought experiment) that if detectors were placed before each slit, the interference pattern would disappear.
Insect resistance transgenic cotton has been produced by inserting a piece of DNA from- a)A virus
- b)A bacterium
- c)A wild relative of cotton
- d)An insect
Correct answer is option 'B'. Can you explain this answer?
Insect resistance transgenic cotton has been produced by inserting a piece of DNA from
a)
A virus
b)
A bacterium
c)
A wild relative of cotton
d)
An insect

|
Syed Saif answered |
Bacillus killing the larvae of flour moths in Germany and published a description of the bacterium and its … Mycogen, another small biotechnology firm, instead cloned Bt genes and expressed them in an alternate host, Pseudomonas fluorescens, a bacterial species common in transgenic cotton.
In a single slit experiment, suppose the slit width is equal to the wavelength of light used. Then- a)Slit image becomes very small
- b)The image of the slit becomes infinitely wide
- c)Diffraction effect cannot be observed
- d)Diffraction pattern becomes wider
Correct answer is option 'B'. Can you explain this answer?
In a single slit experiment, suppose the slit width is equal to the wavelength of light used. Then
a)
Slit image becomes very small
b)
The image of the slit becomes infinitely wide
c)
Diffraction effect cannot be observed
d)
Diffraction pattern becomes wider
|
|
Om Desai answered |
This is because for maximum diffraction the slit width always equals to the wavelength of light.
The phenomena diffraction can take place in sound waves.- a)Yes
- b)No
- c)Only Interference
- d)Under certain conditions only
Correct answer is option 'A'. Can you explain this answer?
The phenomena diffraction can take place in sound waves.
a)
Yes
b)
No
c)
Only Interference
d)
Under certain conditions only
|
|
Rohan Singh answered |
YES, Sound waves, on the other hand, are longitudinal, meaning that they oscillate parallel to the direction of their motion. Since there is no component of a sound wave's oscillation that is perpendicular to its motion, sound waves cannot be polarized
Which of the following undergoes largest diffraction?- a)Ultraviolet light
- b)Infra red light
- c)Radio waves
- d)Y – rays
Correct answer is option 'C'. Can you explain this answer?
Which of the following undergoes largest diffraction?
a)
Ultraviolet light
b)
Infra red light
c)
Radio waves
d)
Y – rays
|
|
Anjana Sharma answered |
Maximum diffraction occurs when size of obstacle is almost equal to wavelength of light wave. Hence maximum diffraction occurs for larger wavelength . As wavelength of radio wave is higher than others, maximum diffraction will occur for it.
Which of the following is an advantage of transgenesis?- a)Study of diseases and gene therapy
- b)Increased production of biological products
- c)Vaccine safety testing
- d)All of the above
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an advantage of transgenesis?
a)
Study of diseases and gene therapy
b)
Increased production of biological products
c)
Vaccine safety testing
d)
All of the above
|
|
Akshitha answered |
Option Dis correct coz all the option true regarding transgensis
A transgenic food crop which may help in solving the problem of night blindness in developing countries is- a)Bt soybean
- b)Flavrsavr tomatoes
- c)Golden rice
- d)Starlink maize
Correct answer is option 'C'. Can you explain this answer?
A transgenic food crop which may help in solving the problem of night blindness in developing countries is
a)
Bt soybean
b)
Flavrsavr tomatoes
c)
Golden rice
d)
Starlink maize
|
|
Chirag Ghosh answered |
Golden Rice as a solution to night blindness
Golden Rice is a genetically modified rice variety that has been developed to help combat vitamin A deficiency, which can lead to night blindness and other health problems in developing countries. The rice is genetically modified to produce beta-carotene, a precursor to vitamin A, which is not naturally found in traditional rice varieties.
How does Golden Rice work?
Golden Rice is genetically engineered to produce beta-carotene in the endosperm of the rice grain, which is the part of the plant that is eaten. Beta-carotene is a pigment that gives fruits and vegetables their orange or yellow color, and it is also a precursor to vitamin A. When humans consume beta-carotene, it is converted to vitamin A in the body.
Why is Golden Rice important?
Vitamin A deficiency is a serious health problem in many developing countries, particularly in Southeast Asia and Africa. According to the World Health Organization, an estimated 250 million preschool children are vitamin A deficient, and it is estimated that between 250,000 and 500,000 malnourished children become blind every year due to vitamin A deficiency. Golden Rice is one potential solution to this problem, as it provides a source of beta-carotene that can be converted to vitamin A in the body.
Conclusion
Golden Rice is a promising solution to the problem of vitamin A deficiency in developing countries, which can lead to night blindness and other health problems. While there is still some controversy surrounding genetically modified crops, Golden Rice has the potential to make a significant impact on the health and well-being of millions of people around the world.
Golden Rice is a genetically modified rice variety that has been developed to help combat vitamin A deficiency, which can lead to night blindness and other health problems in developing countries. The rice is genetically modified to produce beta-carotene, a precursor to vitamin A, which is not naturally found in traditional rice varieties.
How does Golden Rice work?
Golden Rice is genetically engineered to produce beta-carotene in the endosperm of the rice grain, which is the part of the plant that is eaten. Beta-carotene is a pigment that gives fruits and vegetables their orange or yellow color, and it is also a precursor to vitamin A. When humans consume beta-carotene, it is converted to vitamin A in the body.
Why is Golden Rice important?
Vitamin A deficiency is a serious health problem in many developing countries, particularly in Southeast Asia and Africa. According to the World Health Organization, an estimated 250 million preschool children are vitamin A deficient, and it is estimated that between 250,000 and 500,000 malnourished children become blind every year due to vitamin A deficiency. Golden Rice is one potential solution to this problem, as it provides a source of beta-carotene that can be converted to vitamin A in the body.
Conclusion
Golden Rice is a promising solution to the problem of vitamin A deficiency in developing countries, which can lead to night blindness and other health problems. While there is still some controversy surrounding genetically modified crops, Golden Rice has the potential to make a significant impact on the health and well-being of millions of people around the world.
In the diffraction of a single slit experiment, if the slit width is half then the width of the central maxima will be- a)Halved
- b)Becomes four times.
- c)Remains the same
- d)Doubled
Correct answer is option 'D'. Can you explain this answer?
In the diffraction of a single slit experiment, if the slit width is half then the width of the central maxima will be
a)
Halved
b)
Becomes four times.
c)
Remains the same
d)
Doubled

|
Amit Kumar answered |
Fringe width is inversly prop to dist betweenslit so it is double
What is the angular spread of the first minimum and the central maximum when diffraction is observed with 540 nm light through a slit of width 1mm?- a)540 x 10-6 rad
- b)1080 x 10-6 rad
- c)270 x 10-6 rad
- d)810 x 10-6 rad
Correct answer is option 'D'. Can you explain this answer?
What is the angular spread of the first minimum and the central maximum when diffraction is observed with 540 nm light through a slit of width 1mm?
a)
540 x 10-6 rad
b)
1080 x 10-6 rad
c)
270 x 10-6 rad
d)
810 x 10-6 rad
|
|
Rahul Kapoor answered |
The condition for the first minimum ,
So the ? is the angular separation between first minimum and the central maximum.
So the correct answer is (A) . 540 x 10-6 rad
The change in diffraction pattern of a single slit, when the monochromatic source of light is replaced by a source of white light will be- a)Diffracted image is not clear
- b)Diffracted image gets dispersed into constituent colours of white light
- c)The image of the slit becomes infinitely wide
- d)Clear colourful fringe pattern
Correct answer is option 'B'. Can you explain this answer?
The change in diffraction pattern of a single slit, when the monochromatic source of light is replaced by a source of white light will be
a)
Diffracted image is not clear
b)
Diffracted image gets dispersed into constituent colours of white light
c)
The image of the slit becomes infinitely wide
d)
Clear colourful fringe pattern

|
Dr Manju Sen answered |
- When a source of white light is used instead of a monochromatic source, the diffracted image of the slit gets dispersed into constituent colours of white light.
- The central maximum will be white and on either side of the central maximum, there will be coloured fringes.
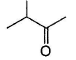
In which of the following compounds, enol form exist? - a)C6H5COCH3
- b)C6H5CHO
- c)

- d)Both (a) and (c)
Correct answer is option 'D'. Can you explain this answer?
In which of the following compounds, enol form exist?
a)
C6H5COCH3
b)
C6H5CHO
c)
d)
Both (a) and (c)
|
|
Preeti Khanna answered |
Both option (a) and option (c) forms enol but option (b) does not form enol.
Which can be deduced correctly regarding keto-enol tautomerism in general?- a)Increasing temperature increases the enol content at equilibrium
- b)Mono-enols are usually more stable than dienols
- c)Enols of ketones are generally more stable than enols of aliphatic aldehydes
- d)Keto-enol taytomerism is catalysed by both acidic and basic catalys
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Which can be deduced correctly regarding keto-enol tautomerism in general?
a)
Increasing temperature increases the enol content at equilibrium
b)
Mono-enols are usually more stable than dienols
c)
Enols of ketones are generally more stable than enols of aliphatic aldehydes
d)
Keto-enol taytomerism is catalysed by both acidic and basic catalys

|
Ishita Deshpande answered |
Increasing temperature increases equilibrium content of less stable enol tautomers.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Enolisation decreases stability, hence introducing two or more enol groups are further more difficult.
Enols of ketones are more substituted at double bond, hence more stable.
Both acid and base catalyses keto-enol tautomerism.
Transgenic plants are the ones- a)Produced by a somatic embryo in artificial medium
- b)Produced after protoplast fusion in artificial medium
- c)Generated by introducing foreign DNA into a cell and regenerating a plant from the cell
- d)Grown in artificial medium after hybridization in the field
Correct answer is option 'C'. Can you explain this answer?
Transgenic plants are the ones
a)
Produced by a somatic embryo in artificial medium
b)
Produced after protoplast fusion in artificial medium
c)
Generated by introducing foreign DNA into a cell and regenerating a plant from the cell
d)
Grown in artificial medium after hybridization in the field
|
|
Awantika Gupta answered |
Transgenic plants are obtained by genetic engineering.
e.g. like Cotton, Flavr savar tomato, Brassica napus, Golden rice etc
e.g. like Cotton, Flavr savar tomato, Brassica napus, Golden rice etc
Only One Option Correct TypeDirection (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. In hexane-2,4-dione, how many different mono-enols are possible?- a)2
- b)3
- c)4
- d)7
Correct answer is option 'D'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-14) This section contains 14 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
In hexane-2,4-dione, how many different mono-enols are possible?
a)
2
b)
3
c)
4
d)
7
|
|
Devanshi Mehta answered |
Possible Mono-enols in Hexane-2,4-dione
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
Hexane-2,4-dione, also known as Acetylacetone, has the following structure:
CH3COCH2COCH3
Mono-enol is the product obtained when one enolizable hydrogen atom of a compound is replaced by a hydroxyl group (-OH).
To determine the number of different mono-enols possible in hexane-2,4-dione, we need to identify the enolizable hydrogen atoms. These are the hydrogen atoms attached to the carbon atoms that are adjacent to the carbonyl groups (-CO-).
In hexane-2,4-dione, there are two such hydrogen atoms, one on each side of the molecule. Therefore, there are two possible enols that can be formed:
- The first enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ketone (-CO-) group on the left side of the molecule. This enol is called the alpha-enol or 1-enol.
CH3COCH=C(OH)CH3
- The second enol is formed by replacing the hydrogen atom attached to the carbon atom adjacent to the ester (-COO-) group on the right side of the molecule. This enol is called the beta-enol or 3-enol.
CH3C(OH)=CHCOCH3
However, each of these enols can exist in two different tautomeric forms: keto and enol. Tautomerism is the phenomenon where a compound exists in two or more isomeric forms that differ only in the position of a hydrogen atom and a double bond.
- The keto form is the one in which the compound has a carbonyl group (-CO-) and no hydroxyl (-OH) group.
- The enol form is the one in which the compound has a double bond (-C=C-) and a hydroxyl (-OH) group.
Therefore, there are four possible mono-enols in hexane-2,4-dione:
- The alpha-keto-enol form, also known as 1,3-diketone form.
- The alpha-hydroxy-ketone form or 1-enol form.
- The beta-keto-enol form, also known as the 3,5-diketone form.
- The beta-hydroxy-ketone form or 3-enol form.
However, each of these forms can also exist as a mixture of both tautomeric forms, keto and enol. Therefore, a total of seven different mono-enols are possible in hexane-2,4-dione:
- Alpha-keto-enol form (1,3-diketone)
- Alpha-enol-keto form (1-enol)
- Alpha-enol-enol form (1-enol)
- Beta-keto-enol form (3,5-diketone)
- Beta-enol-keto form (3-enol)
- Beta-enol-enol form (3-enol)
- Beta-enol-enol-keto form (3-enol)
Therefore, the correct answer is option D, seven.
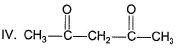
What is the correct order of equilibrium enol content of the following compounds?I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH- a)I > II > III > IV
- b)I > IV > III > II
- c)IV > II > III > I
- d)III > IV > II > I
Correct answer is option 'D'. Can you explain this answer?
What is the correct order of equilibrium enol content of the following compounds?
I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
a)
I > II > III > IV
b)
I > IV > III > II
c)
IV > II > III > I
d)
III > IV > II > I

|
Asha Nair answered |
A 1,3-diketo compound forms more stable enol than a monocarbonyls. Also ester group forms less stable enol than carbonyls. Hence, III, a 1 , 3-diketo ne form s highest enol content while I (monocarbonyl) forms least enol content at equilibrium.
The diffraction of light is not easily noticed if- a)The wave length of visible range is small
- b)Light waves are transverse and longitudinal in nature
- c)Size of ordinary objects are small
- d)Apparatus are needed for noticing
Correct answer is option 'A'. Can you explain this answer?
The diffraction of light is not easily noticed if
a)
The wave length of visible range is small
b)
Light waves are transverse and longitudinal in nature
c)
Size of ordinary objects are small
d)
Apparatus are needed for noticing

|
Prakhar Maheshwari answered |
Actually, diffraction occurs all the time. But when the aperture/wavelength ratio is large, the spacing between fringes becomes too small, and the diffractive effects are unnoticeable.
The correct statement(s) regarding hydrates of aldehyde and ketone is/are- a)Usually hydrates have lower thermodynamic stability than anhydrous form
- b)Hydrate content of acetone is greater in water than in hexane
- c)CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
- d)C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
Correct answer is option 'A,B,C'. Can you explain this answer?
The correct statement(s) regarding hydrates of aldehyde and ketone is/are
a)
Usually hydrates have lower thermodynamic stability than anhydrous form
b)
Hydrate content of acetone is greater in water than in hexane
c)
CH3CHO when treated with H2O18 in acidic medium, gets converted into CH3CHO18
d)
C6H5CHO has greater hydrate content than p-nitrobenzaldehyd
|
|
Nisha Kulkarni answered |
Hydrates of aldehydes and ketones are less stable than anhydrous form (gem diols are unstable).
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
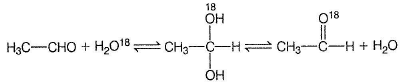
Hydrates form H-bonds with water, hence hydrate content is more in water than in hexane.
This exchange occur via hydrate.
If butanone is treated with D2O18/DCI, isotopic exchange occur. What maximum gain in molecular mass is possible in the present case?
Correct answer is '7'. Can you explain this answer?
If butanone is treated with D2O18/DCI, isotopic exchange occur. What maximum gain in molecular mass is possible in the present case?

|
Asha Nair answered |
Gain of 7 units in molar mass is observed, five units due to 'D' and tw o units due to `O18'.
What is the major product in the following reaction?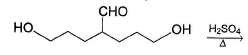
- a)
- b)
- c)
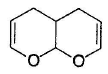
- d)
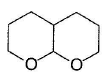
Correct answer is option 'D'. Can you explain this answer?
What is the major product in the following reaction?
a)
b)
c)
d)

|
Pragati Choudhury answered |
Acetal is form ed by cyclisation
Comprehension Type Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageIn general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.Q. The major final product in the following reaction is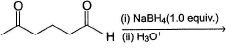
- a)

- b)

- c)
- d)Mixture of (b) and (c)
Correct answer is option 'C'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 20-22) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Q.
The major final product in the following reaction is
a)
b)
c)
d)
Mixture of (b) and (c)

|
Ameya Tiwari answered |
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.Consider the following reaction,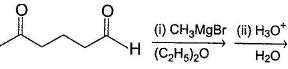 Q. Major product is
Q. Major product is - a)
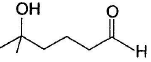
- b)

- c)
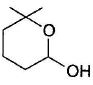
- d)
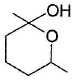
Correct answer is option 'D'. Can you explain this answer?
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Consider the following reaction,
Q.
Major product is
a)
b)
c)
d)

|
Sankar Chakraborty answered |
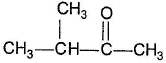
One or More than One Options Correct TypeDirection (Q. Nos. 15-19) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q.Which of the following has (have) more than one enol tautomers?- a)
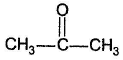
- b)
- c)CH3CH2CHO
- d)
Correct answer is option 'B,C'. Can you explain this answer?
One or More than One Options Correct Type
Direction (Q. Nos. 15-19) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which of the following has (have) more than one enol tautomers?
a)
b)
c)
CH3CH2CHO
d)

|
Sai Chakraborty answered |
Arrange the following in the increasing order of stability of their most stable enol.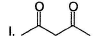
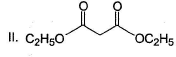
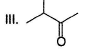
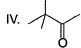
- a) I < II < III < IV
- b)IV < III < II < I
- c)II < I < IV < III
- d)III < IV < II < I
Correct answer is option 'B'. Can you explain this answer?
Arrange the following in the increasing order of stability of their most stable enol.
a)
I < II < III < IV
b)
IV < III < II < I
c)
II < I < IV < III
d)
III < IV < II < I

|
Sai Chakraborty answered |
Diketo (I) forms highest enol content due to stabilisation of enol by intermolecular H-bonding. Electron donating resonance effect by ester group slightly decreases enol content.
For which of the following equilibrium, Kc is greater than 1?- a)
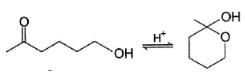
- b)
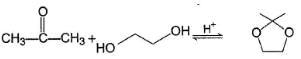
- c)
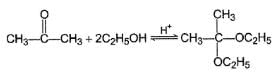
- d)
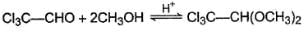
Correct answer is option 'A,B'. Can you explain this answer?
For which of the following equilibrium, Kc is greater than 1?
a)

b)

c)

d)
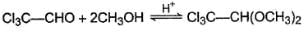

|
Kavya Das answered |
Five and six-membered cyclic hemiacetals are more stable than its hydroxy aldehydes/ketones, predominate at equilibrium. Cyclic acetals are highly stable, predominate at equilibrium.
Arrange the following in the increasing order of hydrate content at equilibrium in aqueous solutionI. H2CO
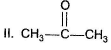
III. C6H5CHO

- a)I < II < III < IV
- b)IV < III < II < I
- c)III < IV < II < I
- d)Ill < IV < I < II
Correct answer is option 'C'. Can you explain this answer?
Arrange the following in the increasing order of hydrate content at equilibrium in aqueous solution
I. H2CO
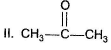
III. C6H5CHO

III. C6H5CHO
a)
I < II < III < IV
b)
IV < III < II < I
c)
III < IV < II < I
d)
Ill < IV < I < II
|
|
Shalini Basu answered |
Due to negligible steric hindrance, form aldehyde form s large hydrate content at equilibrium. Also electron withdrawing group in (IV) increases hydrate content compared to III.
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.Q. How the following transformation can be best brought about?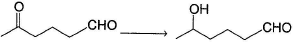
- a){(CH3)2CHO}3AI;H3O+
- b)
 then NaBH4 followed by H3O+
then NaBH4 followed by H3O+ - c)H2/Ni; high p and T
- d)N2H4/NaOH/Heat
Correct answer is option 'B'. Can you explain this answer?
In general, ketones are less reactive than aldehydes in nucleophilic addition reaction, for both steric and electronic reasons.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Hence, if a keto-aldehyde is treated with nucleophilic reagent, reaction occur first at aldehyde group.
Q.
How the following transformation can be best brought about?
a)
{(CH3)2CHO}3AI;H3O+
b)
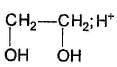
then NaBH4 followed by H3O+
c)
H2/Ni; high p and T
d)
N2H4/NaOH/Heat

|
Pragati Choudhury answered |
Aldehyde being more reactive, protected first by acetal formation followed by reduction of ketone group.
Arrange the following in the increasing order of acidic strength I. H2CO
II. CH3CHO
III. C6H5CH2CHO
- a)I < II < III < IV
- b)IV < III < II < I
- c)III < IV < II < I
- d)III < IV < I < II
Correct answer is option 'A'. Can you explain this answer?
Arrange the following in the increasing order of acidic strength
I. H2CO
II. CH3CHO
III. C6H5CH2CHO

II. CH3CHO
III. C6H5CH2CHO
a)
I < II < III < IV
b)
IV < III < II < I
c)
III < IV < II < I
d)
III < IV < I < II

|
Maitri Sharma answered |
IV is most acidic as its conjugate base is resonance stabilised by two carbonyl groups, followed by III whose conjugate base is resonance stabilised by a carbonyl group and phenyi ring.
For which of the following equilibrium, Kc < 1 ?- a)
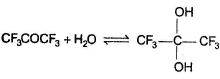
- b)
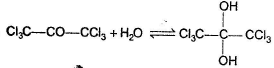
- c)
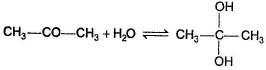
- d)
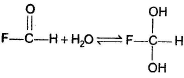
Correct answer is option 'C'. Can you explain this answer?
For which of the following equilibrium, Kc < 1 ?
a)
b)
c)
d)

|
Nidhi Nambiar answered |
In the absence of any special stability factor, a hydrate of ketone is highly unstable.
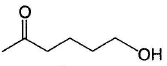
Consider the following reaction,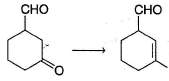 Q. The appropriatensequence of reagent that can best bring about the above conversion is
Q. The appropriatensequence of reagent that can best bring about the above conversion is- a)(i) CH3MgBr (1.0 equiv.) (ii) Conc. H2SO4/ Heat
- b)(i) CH3Li(1.0 equiv.)(ii)Conc. H2SO4/Heat
- c)(i) C2H5OH (2.0 equiv.); H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat
- d)(i) HOCH2CH2OH; H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat (iv) H3O+/H2O
Correct answer is option 'D'. Can you explain this answer?
Consider the following reaction,
Q.
The appropriatensequence of reagent that can best bring about the above conversion is
a)
(i) CH3MgBr (1.0 equiv.) (ii) Conc. H2SO4/ Heat
b)
(i) CH3Li(1.0 equiv.)(ii)Conc. H2SO4/Heat
c)
(i) C2H5OH (2.0 equiv.); H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat
d)
(i) HOCH2CH2OH; H+ (ii) CH3MgBr (iii) Conc. H2SO4/Heat (iv) H3O+/H2O

|
Dishani Kulkarni answered |
Which of the following observation does not establish the existence of keto-enol tautomerism in acetone?- a)When treated with DCI in D2O, hexadeuterated acetone is recovered
- b)When treated with D2O/NaOD, hexadeuterated acetone is recovered
- c)When treated with dil. H2SO4 in H2O18,
 is obtained
is obtained - d)All of the above establishes keto-enol tautomerism
Correct answer is option 'C'. Can you explain this answer?
Which of the following observation does not establish the existence of keto-enol tautomerism in acetone?
a)
When treated with DCI in D2O, hexadeuterated acetone is recovered
b)
When treated with D2O/NaOD, hexadeuterated acetone is recovered
c)
When treated with dil. H2SO4 in H2O18, 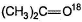 is obtained
is obtained
d)
All of the above establishes keto-enol tautomerism

|
Pragati Choudhury answered |
The above exchange occur due to existence of hydrate equilibrium.
Among the following compounds, one that will not show keto-enol tautomerism is- a)

- b)
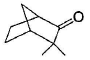
- c)
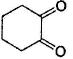
- d)
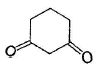
Correct answer is option 'B'. Can you explain this answer?
Among the following compounds, one that will not show keto-enol tautomerism is
a)
b)
c)
d)

|
Kavya Das answered |
sp2 hybridisation is very less stable at bridgehead carbon of a bicyclic compound.
Hemiacetals are usually unstable whereas acetals are stable. Which has the most stable hemiacetal?- a)
- b)
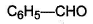
- c)

- d)
Correct answer is option 'D'. Can you explain this answer?
Hemiacetals are usually unstable whereas acetals are stable. Which has the most stable hemiacetal?
a)
b)
c)
d)

|
Kavya Das answered |
If a hemiacetal is five or six membered cyclic, they are stable.
Consider the reaction below,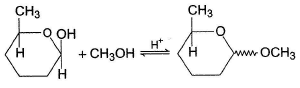 The correct statement(s) regarding the above process is/are
The correct statement(s) regarding the above process is/are- a)Equilibrium favours product
- b)Pair of enantiomers of acetals are formed
- c)Pair of diastereomers of acetals are formed
- d)Equilibrium favours reactant
Correct answer is option 'A,C'. Can you explain this answer?
Consider the reaction below,
The correct statement(s) regarding the above process is/are
a)
Equilibrium favours product
b)
Pair of enantiomers of acetals are formed
c)
Pair of diastereomers of acetals are formed
d)
Equilibrium favours reactant

|
Ameya Tiwari answered |
Product is an acetal, more stable than hemiacetal reactant. Acetal is formed via carbocation, both diastereomers are formed.
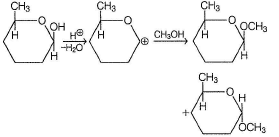
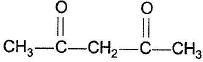
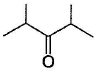
Which of the following has greater enol content than keto counter part?- a)C6H5—CH2CHO
- b)
- c)
- d)
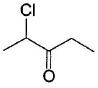
Correct answer is option 'B'. Can you explain this answer?
Which of the following has greater enol content than keto counter part?
a)
C6H5—CH2CHO
b)
c)
d)

|
Pragati Choudhury answered |
It is 1, 3-dik eto compound, forms stable enol.
Chapter doubts & questions for January Week 1 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of January Week 1 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup










