All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of February Week 2 for NEET Exam
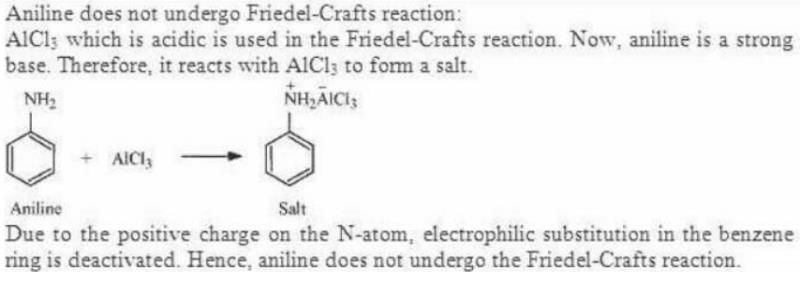
Hoffmann Bromamide Degradation reaction is shown by __________.- a) ArNH2
- b)ArCONH2
- c)ArNO2
- d)ArCH2NH2
Correct answer is option 'B'. Can you explain this answer?
Hoffmann Bromamide Degradation reaction is shown by __________.
a)
ArNH2
b)
ArCONH2
c)
ArNO2
d)
ArCH2NH2
|
|
Hansa Sharma answered |
The Correct answer is option B
Hoffman bromamide degradation reaction is shown by ArCONH2.
Where the aryl amide is converted to aryl amine in the presence of Br2 and NaOH
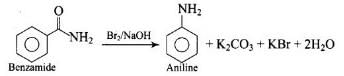
Hoffman bromamide degradation reaction is shown by ArCONH2.
Where the aryl amide is converted to aryl amine in the presence of Br2 and NaOH

Which of the following has highest boiling point?- a)(C2H5)2NH
- b)C2H5N(CH3)2
- c)C2H5NH(CH3)2
- d)n-C4H9NH2
Correct answer is option 'D'. Can you explain this answer?
Which of the following has highest boiling point?
a)
(C2H5)2NH
b)
C2H5N(CH3)2
c)
C2H5NH(CH3)2
d)
n-C4H9NH2
|
|
Krishna Iyer answered |
The correct answer is Option D.
Because C4H9NH2 is primary amine and can form hydrogen bonding more than secondary amine (C2H5)2NH and tertiary amine C2H5N(CH3)2 . More hydrogen bonding leads to strong bonding between the molecules, hence increases the boiling point.
Which of the following amine liberates nitrogen gas on reaction with HNO2 ?- a)(CH3)2NH
- b)(CH3)3 N
- c)C6H5 NH2
- d)CH3NH2
Correct answer is option 'D'. Can you explain this answer?
Which of the following amine liberates nitrogen gas on reaction with HNO2 ?
a)
(CH3)2NH
b)
(CH3)3 N
c)
C6H5 NH2
d)
CH3NH2
|
|
Krishna Iyer answered |
The correct answer is Option D.
Only primary amines liberate nitrogen gas on reaction with HNO2.
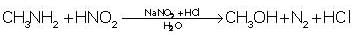
Only primary amines liberate nitrogen gas on reaction with HNO2.
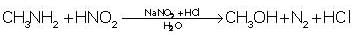
A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time in years, after which one fourth of the material remains is- a)4860
- b)2340
- c)1080.0
- d)3240
Correct answer is option 'C'. Can you explain this answer?
A radioactive material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time in years, after which one fourth of the material remains is
a)
4860
b)
2340
c)
1080.0
d)
3240
|
|
Nikita Singh answered |
Since, from Rutherford-Soddy law, the number of atoms left after half-lives is given by
N=N0(1/2)n
where, N0 is the original number of atoms.
The number of half-lives, n= time of decay/effective half−life
Relation between effective disintegration constant (λ) and half-life (T)
λ=ln2/T
∴λ1+λ2= (ln2/ T1)+ (ln2/ T2)
Effective half-life,
1/T=1/T1+1/T2=(1/1620)+(1/810)
1/T=1+2/1620 ⇒T=540yr
∴n=T/540
∴N=N0(1/2)t/540⇒N/N0=(1/2)2=(1/2)t/540
⇒t/540=2⇒t=2×540=1080yr
N=N0(1/2)n
where, N0 is the original number of atoms.
The number of half-lives, n= time of decay/effective half−life
Relation between effective disintegration constant (λ) and half-life (T)
λ=ln2/T
∴λ1+λ2= (ln2/ T1)+ (ln2/ T2)
Effective half-life,
1/T=1/T1+1/T2=(1/1620)+(1/810)
1/T=1+2/1620 ⇒T=540yr
∴n=T/540
∴N=N0(1/2)t/540⇒N/N0=(1/2)2=(1/2)t/540
⇒t/540=2⇒t=2×540=1080yr
Cadmium rods are used in a nuclear reactor for- a)absorbing neutrons
- b)speeding up slow neutrons
- c)regulating the power level of the reactor.
- d)slowing down fast neutrons
Correct answer is option 'A'. Can you explain this answer?
Cadmium rods are used in a nuclear reactor for
a)
absorbing neutrons
b)
speeding up slow neutrons
c)
regulating the power level of the reactor.
d)
slowing down fast neutrons

|
Awantika Gupta answered |
Cadmium and boron rod both are used in cotroling the reactivity of uranium means slow down the rate of fission...
All nuclides with same mass number A are called- a)isobars
- b)isoclines
- c)isotones
- d)isotopes
Correct answer is option 'A'. Can you explain this answer?
All nuclides with same mass number A are called
a)
isobars
b)
isoclines
c)
isotones
d)
isotopes
|
|
Rocky Handsome answered |
Isobars are atoms of different elements with the same mass number but different atomic numbers.
• Isotones are atomic nuclei with the same number of neutrons (N) and different number of protons(Z)
• Isotones are atomic nuclei with the same number of neutrons (N) and different number of protons(Z)
90% of a radioactive sample is left undisintegrated after time τ has elapsed, what percentage of initial sample will decay in a total time2τ?- a)9%
- b)38%
- c)19%
- d)62%
Correct answer is option 'C'. Can you explain this answer?
90% of a radioactive sample is left undisintegrated after time τ has elapsed, what percentage of initial sample will decay in a total time2τ?
a)
9%
b)
38%
c)
19%
d)
62%
|
|
Krishna Iyer answered |
Given that 90% is left un-decayed after time 't'.
Hence, 10% decays in time 't'.
Initially assume that the amount of substance is 'x'
After time 't' 10% is decayed.
i.e. Amount of substance left =0.9x
After further time 't' another 10% is decayed.
i.e. 0.1×0.9x is decayed
Leaving behind 0.81x.
Hence after time 2t we see that 0.19x has decayed, which is 19%.
Hence, 10% decays in time 't'.
Initially assume that the amount of substance is 'x'
After time 't' 10% is decayed.
i.e. Amount of substance left =0.9x
After further time 't' another 10% is decayed.
i.e. 0.1×0.9x is decayed
Leaving behind 0.81x.
Hence after time 2t we see that 0.19x has decayed, which is 19%.
Which of the following are also called lungs of our planet?
- a)Himalayas
- b)Amazonian rainforests
- c)Mediterranean Basin
- d)Western Ghats
Correct answer is option 'B'. Can you explain this answer?
Which of the following are also called lungs of our planet?
a)
Himalayas
b)
Amazonian rainforests
c)
Mediterranean Basin
d)
Western Ghats

|
Ayush Choudhury answered |
Amzonian rain forests are called as lungs of forest because it contains different kinds of vegetation that purify the atmosphere of the earth.
α-rays are- a)helium nuclei
- b)heavy nuclei
- c)lithium nuclei
- d)hydrogen nuclei
Correct answer is option 'A'. Can you explain this answer?
α-rays are
a)
helium nuclei
b)
heavy nuclei
c)
lithium nuclei
d)
hydrogen nuclei
|
|
Ræjû Bhæï answered |
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways.
At a given time there are 25% undecayed nuclei in a sample. After 10 seconds number of undecayed nuclei reduces to 12.5%. Then mean life of the nuclei will be about- a)22 sec
- b)10 sec
- c)12 sec
- d)15 sec
Correct answer is option 'D'. Can you explain this answer?
At a given time there are 25% undecayed nuclei in a sample. After 10 seconds number of undecayed nuclei reduces to 12.5%. Then mean life of the nuclei will be about
a)
22 sec
b)
10 sec
c)
12 sec
d)
15 sec
|
|
Lavanya Menon answered |
Half-life of radioactive sample, i.e., the time in which the number of undecayed nuclei becomes half (T) is 10 s.
Mean life, τ=T/loge2=10s/0.693=1.443×10=14.43s ≈ 15s
Mean life, τ=T/loge2=10s/0.693=1.443×10=14.43s ≈ 15s
The half life of radon is 3.8 days. After how many days will only one twentieth of a radon sample be left over?
a)10.00 daysb)5.45 daysc)15.45 daysd)16.45 daysCorrect answer is option 'D'. Can you explain this answer?
|
|
Suresh Iyer answered |
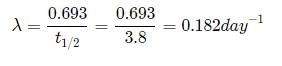 Let the initial amount of radon be N0 and the amount left after t days be N which is equal to N0/2
Let the initial amount of radon be N0 and the amount left after t days be N which is equal to N0/2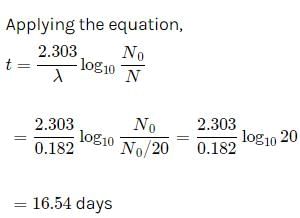
If all the members of a host species die then all its unique parasites also die off, representing:- a)biological control
- b)co-extinction
- c)conservation
- d)extinction
Correct answer is option 'B'. Can you explain this answer?
If all the members of a host species die then all its unique parasites also die off, representing:
a)
biological control
b)
co-extinction
c)
conservation
d)
extinction

|
Diya Datta answered |
If all members fo a host species die the parasite that obtain their food from the particular host also die off due to lack of food. This represent co-existence of species.
What is the main source of energy of the sun?- a)Nuclear fission of heavier unstable elements in the sun
- b)Combustion of pure carbon present in the sun
- c)Gravitational energy liberated during the slow contraction of the sun.
- d)Nuclear fusion of lighter elements in the sun.
Correct answer is option 'D'. Can you explain this answer?
What is the main source of energy of the sun?
a)
Nuclear fission of heavier unstable elements in the sun
b)
Combustion of pure carbon present in the sun
c)
Gravitational energy liberated during the slow contraction of the sun.
d)
Nuclear fusion of lighter elements in the sun.

|
Kanika S answered |
Option d
When H and He in sun undergoes fusion large amount of heat is released.
When H and He in sun undergoes fusion large amount of heat is released.
The average number of neutrons released by the fission of one uranium atom is
a)3.0b)2c)2.5d)1Correct answer is option 'C'. Can you explain this answer?
|
|
Bhanu Saini answered |
Fission result in the production of typically 2 or 3 neutron so on the average about 2.5 neutron released per unit. so correct answer is option a
for option c one uranium atom split into one barium and one krypton atom releasing 3 neutron.
but in this question average is asking so according to me and books 2.5 is correct
for option c one uranium atom split into one barium and one krypton atom releasing 3 neutron.
but in this question average is asking so according to me and books 2.5 is correct
Nuclear mass M is found to be- a)always greater than total mass of its individual protons and neutrons
- b)always equal to the total mass of its individual neutrons
- c)always equal to the total mass of its individual protons and neutrons
- d)always less than total mass of its individual protons and neutrons
Correct answer is option 'D'. Can you explain this answer?
Nuclear mass M is found to be
a)
always greater than total mass of its individual protons and neutrons
b)
always equal to the total mass of its individual neutrons
c)
always equal to the total mass of its individual protons and neutrons
d)
always less than total mass of its individual protons and neutrons
|
|
Ritu Singh answered |
The actual mass is always less than the sum of the individual masses of the constituent protons and neutrons because energy is removed when the nucleus is formed. This energy has mass, which is removed from the total mass of the original particles.
What percentage of the mass of an atom is concentrated in the nucleus?- a)79.9%
- b)99.9%
- c)66.9%
- d)50.9%
Correct answer is option 'B'. Can you explain this answer?
What percentage of the mass of an atom is concentrated in the nucleus?
a)
79.9%
b)
99.9%
c)
66.9%
d)
50.9%
|
|
Jyoti Kapoor answered |
More than 99.99% of the mass of any atom is concentrated in its nucleus. If the mass of protons and neutrons (which are in the nucleus of every atom) is approximately one (1) atomic mass unit, then the relative mass of an electron is 0.0005 atomic mass units.
Which one of the following is a tertiary amine?- a)Ethylamine
- b)Triethylamine
- c)Aniline
- d)Diethylamine
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is a tertiary amine?
a)
Ethylamine
b)
Triethylamine
c)
Aniline
d)
Diethylamine
|
|
Suresh Iyer answered |
The correct answer is Option B.
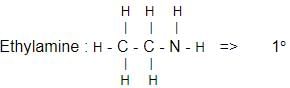
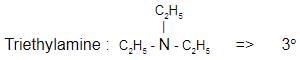
Aniline :
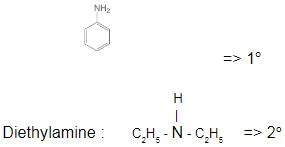
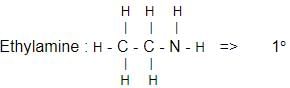
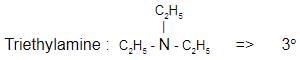
Aniline :

Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is - a)1 /3
- b)1 /2
- c)1/8
- d)1 /4
Correct answer is option 'C'. Can you explain this answer?
Plutonium decays with a half-life of 24000 years. If the plutonium is stored for 72000 years, then the fraction of plutonium that remains is
a)
1 /3
b)
1 /2
c)
1/8
d)
1 /4
|
|
Mira Sharma answered |
The amount of plotinium after a time period of 72000 if the half life is 24000 will be
the initial amount x would be reduced to x/2 , in 24000 yrs
then it would lessen to x/4 in the next 24000yrs
and then to x/8 in the next 24000 yrs
that is it will reduce to x/8 in the next 72000yrs starting from x .
In the mass number range A = 30 to 170, the binding energy per nucleon is- a)decreases with increasing A
- b)increases linearly with A
- c)decreases linearly with A
- d)nearly constant
Correct answer is option 'D'. Can you explain this answer?
In the mass number range A = 30 to 170, the binding energy per nucleon is
a)
decreases with increasing A
b)
increases linearly with A
c)
decreases linearly with A
d)
nearly constant
|
|
Shraddha Choudhury answered |
Binding energy per nucleon in the mass number range A = 30 to 170
The binding energy per nucleon is the energy required to separate a nucleus into its constituent nucleons. It is a measure of the stability of the nucleus, and it depends on the mass number of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
Explanation:
The binding energy per nucleon is given by the formula:
BE/A = (ZmH + NmN - M)/A
where BE is the binding energy, Z is the atomic number, N is the number of neutrons, mH is the mass of a hydrogen atom, mN is the mass of a neutron, and M is the mass of the nucleus.
In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant because the nuclear force between nucleons is nearly constant. This means that the energy required to separate a nucleon from the nucleus is nearly constant in this range.
The nuclear force between nucleons is a strong force that holds the nucleus together. It is a short-range force that depends on the distance between nucleons. In the mass number range A = 30 to 170, the distance between nucleons is nearly constant, and so the nuclear force is nearly constant.
Therefore, the binding energy per nucleon is nearly constant in this range because the nuclear force is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
The binding energy per nucleon is the energy required to separate a nucleus into its constituent nucleons. It is a measure of the stability of the nucleus, and it depends on the mass number of the nucleus. In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
Explanation:
The binding energy per nucleon is given by the formula:
BE/A = (ZmH + NmN - M)/A
where BE is the binding energy, Z is the atomic number, N is the number of neutrons, mH is the mass of a hydrogen atom, mN is the mass of a neutron, and M is the mass of the nucleus.
In the mass number range A = 30 to 170, the binding energy per nucleon is nearly constant because the nuclear force between nucleons is nearly constant. This means that the energy required to separate a nucleon from the nucleus is nearly constant in this range.
The nuclear force between nucleons is a strong force that holds the nucleus together. It is a short-range force that depends on the distance between nucleons. In the mass number range A = 30 to 170, the distance between nucleons is nearly constant, and so the nuclear force is nearly constant.
Therefore, the binding energy per nucleon is nearly constant in this range because the nuclear force is nearly constant. This means that the stability of the nucleus is nearly constant in this range.
When we compare the relationship between species richness and area for wide variety of taxa, the graph appears to be a :- a)cubic parabola
- b)rectangular hyperbola
- c)cubic hyperbola
- d)rectangular parabola
Correct answer is option 'D'. Can you explain this answer?
When we compare the relationship between species richness and area for wide variety of taxa, the graph appears to be a :
a)
cubic parabola
b)
rectangular hyperbola
c)
cubic hyperbola
d)
rectangular parabola

|
Prasenjit Pillai answered |
When a graph is drawn to compare the relationship between species richness and area for wide variety of texa, the graph appears to be a rectangular hyperbola.
If we say, India has about 50,000 type of rice and 1000 types of varieties of mango what level of diversity it indicates:- a)community diversity
- b)ecosystem diversity
- c)genetic diversity
- d)species diversity
Correct answer is option 'C'. Can you explain this answer?
If we say, India has about 50,000 type of rice and 1000 types of varieties of mango what level of diversity it indicates:
a)
community diversity
b)
ecosystem diversity
c)
genetic diversity
d)
species diversity

|
Naveen Menon answered |
India has about 50,000 type of rice and 1000 types of varieties of mango. This thigh level of diversity indicates very high genetic diversity and one of the largest in the world.
When we will move away from the equator towards poles, we will find:- a)a gradual decrease in the species diversity
- b)a gradual increase in the ecosystem diversity
- c)a gradual increase in the species diversity
- d)no change in the species diversity
Correct answer is option 'A'. Can you explain this answer?
When we will move away from the equator towards poles, we will find:
a)
a gradual decrease in the species diversity
b)
a gradual increase in the ecosystem diversity
c)
a gradual increase in the species diversity
d)
no change in the species diversity

|
Krish Patel answered |
When we will move away from the equator towards poles there is a gradual decrease in the species diversity. This is because climatic condition becomes gradually adverse as we move from equator to poles.
In a wetland the primary factor controlling the environment and the associated plant and animal life will be:- a)water
- b)temperature
- c)soil
- d)light
Correct answer is option 'A'. Can you explain this answer?
In a wetland the primary factor controlling the environment and the associated plant and animal life will be:
a)
water
b)
temperature
c)
soil
d)
light

|
Akshat Chavan answered |
The area that is saturated with water is called wetland. In wetland the primary factor controlling the environment and the associated plant and animal life will be water.
The nuclei of isotopes of a given element contain the same number of- a)neutrinos
- b)protons
- c)neutrons
- d)positrons
Correct answer is option 'B'. Can you explain this answer?
The nuclei of isotopes of a given element contain the same number of
a)
neutrinos
b)
protons
c)
neutrons
d)
positrons
|
|
Varanasi Sai Srinivasa K answered |
Atom of same element, contain same number of protons, they differ in number of neutrons .
This is known as isotope .
Therefore we can conclude that answer is [ B ]
This is known as isotope .
Therefore we can conclude that answer is [ B ]
Amines are basic in nature as they have:- a)Replaceable H atom
- b)A lone pair of electrons on nitrogen
- c)Replaceable N-H group
- d)A hydroxyl group in the molecule
Correct answer is option 'B'. Can you explain this answer?
Amines are basic in nature as they have:
a)
Replaceable H atom
b)
A lone pair of electrons on nitrogen
c)
Replaceable N-H group
d)
A hydroxyl group in the molecule

|
Rishita Rai answered |
Correct option is b because as there is lone pair on nitrogen they possess a lone pair that can be donated that's why amines are bases
The chemical behavior of an atom depends upon- a)the number of neutrons in its nucleus
- b)the number of nucleons in its nucleus.
- c)the number of electrons orbiting around its nucleus
- d)the number of protons in its nucleus
Correct answer is option 'C'. Can you explain this answer?
The chemical behavior of an atom depends upon
a)
the number of neutrons in its nucleus
b)
the number of nucleons in its nucleus.
c)
the number of electrons orbiting around its nucleus
d)
the number of protons in its nucleus

|
Kuheli Sengupta answered |
This is because in most chemical reactions only electrons participate. the nucleons have little role in chemical reactions
Nuclear fusion is possible- a)only between light nuclei
- b)only between heavy nuclei
- c)between both light and heavy nuclei
- d)only between nuclei which are stable against β-decay
Correct answer is option 'A'. Can you explain this answer?
Nuclear fusion is possible
a)
only between light nuclei
b)
only between heavy nuclei
c)
between both light and heavy nuclei
d)
only between nuclei which are stable against β-decay
|
|
Harshit Agrawal answered |
In nuclear fusion, two or more small nuclei combine to form a single larger nucleus, a neutron, and a tremendous amount of energy. Nuclear fusion of hydrogen to form helium occurs naturally in the sun and other stars. It takes place only at extremely high temperatures.
B210has a half life of 5 days. The time taken for seven-eighth of a sample to decay is- a)10 days
- b)20 days
- c)3.4 days
- d)15 days
Correct answer is option 'D'. Can you explain this answer?
B210has a half life of 5 days. The time taken for seven-eighth of a sample to decay is
a)
10 days
b)
20 days
c)
3.4 days
d)
15 days
|
|
Srishti Chavan answered |
Half-life of Bi210=5 days
∴k= 0.693/(t1/2) =(0.693/5) day−1
Using k=(2.303/t) log (a/a-x)
(where a = a0, (let) ⇒x=7/8 a0, t is time taken in decay and k is rate constant)
We get, t=(2.303×5/0.693)log a0/(1/8)a0
= (2.303×5/0.693) log8=15days
∴k= 0.693/(t1/2) =(0.693/5) day−1
Using k=(2.303/t) log (a/a-x)
(where a = a0, (let) ⇒x=7/8 a0, t is time taken in decay and k is rate constant)
We get, t=(2.303×5/0.693)log a0/(1/8)a0
= (2.303×5/0.693) log8=15days
Introducing exotic species into new areas will:
i) increase competition for food & space.
ii) introduce diseases
iii) improve habitat
iv) lead to extinction of native species- a)only iv is correct.
- b)only ii, iii & iv are correct.
- c)only i, ii & iv are correct.
- d)all the above are correct.
Correct answer is option 'C'. Can you explain this answer?
Introducing exotic species into new areas will:
i) increase competition for food & space.
ii) introduce diseases
iii) improve habitat
iv) lead to extinction of native species
i) increase competition for food & space.
ii) introduce diseases
iii) improve habitat
iv) lead to extinction of native species
a)
only iv is correct.
b)
only ii, iii & iv are correct.
c)
only i, ii & iv are correct.
d)
all the above are correct.
|
|
Geetika Shah answered |
The impacts of introducing a non-native or invasive species to an ecosystem will vary depending on a number of factors.
In some instances, the introduced species may not survive. If there is no ecological niche for the species to fill or the species cannot adapt to fill a different ecological niche, the species will likely go extinct relatively quickly at the local level.
However, if the species is a generalist, or a species able to thrive in a variety of environments and consume many food sources, that species will likely do well. If the ecosystem has reached its stable state, this means that the invasive species will have to replace a native species. No two species can share the same ecological niche, thus one will be better adapted and survive. If the invasive species is better adapted, it will out compete the native species.
If the species reproduces quickly, it is also more likely to thrive in a new ecosystem. If it can reproduce and grow faster than its competitor, it will eventually out compete that species.
Typically, invasive species harm an ecosystem. For example, the Burmese python is found in the US but it isn't supposed to be here. These snakes were likely released by humans and were pets at one time. The environment is suitable for them and they have adapted to the area.
Introducing a new species can also introduce any diseases that species has. These new diseases can spread to other native species and negatively affect them.
Introducing exotic species into new areas will increase competition for food and space. Sometimes, exotic species brings disease along with them. Exotic species in new area do not lead to extinction of native species.
Which one of the following is the weakest base in gaseous phase?- a)Triethyl amine
- b)Ethyl amine
- c)Diethyl amine
- d)Ammonia
Correct answer is option 'D'. Can you explain this answer?
Which one of the following is the weakest base in gaseous phase?
a)
Triethyl amine
b)
Ethyl amine
c)
Diethyl amine
d)
Ammonia
|
|
Rajat Kapoor answered |
As the number of alkyl group increases, due to +I effect the basicity of amines increases.
Choose the WRONG statement. A thermonuclear fusion reactor is better than a fission reactor for the following reason:- a)For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
- b)The fuel required for fusion is readily available in abundance from seawater.
- c)A fusion reaction can be much more easily controlled than a fission
- d)A fusion reaction produces almost no radioactive waste.
Correct answer is option 'B'. Can you explain this answer?
Choose the WRONG statement. A thermonuclear fusion reactor is better than a fission reactor for the following reason:
a)
For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
b)
The fuel required for fusion is readily available in abundance from seawater.
c)
A fusion reaction can be much more easily controlled than a fission
d)
A fusion reaction produces almost no radioactive waste.
|
|
Nisha Kulkarni answered |
Explanation:
The wrong statement among the given options is option B: "The fuel required for fusion is readily available in abundance from seawater."
Reason:
- While it is correct that a thermonuclear fusion reactor is better than a fission reactor for several reasons, including higher energy release and less radioactive waste production, the availability of fuel from seawater is not accurate.
- Fusion reactions require isotopes of hydrogen, such as deuterium and tritium, as fuel. Deuterium can be extracted from seawater, but tritium is a radioactive isotope that is not naturally abundant and needs to be produced artificially.
- Tritium can be produced by exposing lithium to neutron radiation, which can be generated by a fission reactor or a fusion reactor itself. However, the process of producing tritium is not as straightforward as extracting deuterium from seawater.
- Tritium is also highly radioactive and has a short half-life, which means it requires careful handling and containment. It cannot be easily stored or transported.
- Therefore, the fuel required for fusion reactions is not readily available in abundance from seawater, as stated in option B.
Correct statements:
a) For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
- This is true. Fusion reactions release a tremendous amount of energy, several times more than fission reactions. The fusion of hydrogen atoms into helium is the same process occurring in the Sun and other stars, which produces immense amounts of energy.
c) A fusion reaction can be much more easily controlled than a fission reaction.
- This is true. Fusion reactions require extremely high temperatures and pressures to sustain, and if these conditions are not maintained, the reaction will cease. This inherent stability makes fusion reactions more easily controllable than fission reactions, which can lead to runaway chain reactions if not properly regulated.
d) A fusion reaction produces almost no radioactive waste.
- This is true. Fusion reactions do not produce long-lived radioactive waste like fission reactions. The only radioactive byproduct of fusion is tritium, which has a relatively short half-life and can be managed safely.
In summary, option B is the wrong statement because the fuel required for fusion reactions is not readily available in abundance from seawater.
The wrong statement among the given options is option B: "The fuel required for fusion is readily available in abundance from seawater."
Reason:
- While it is correct that a thermonuclear fusion reactor is better than a fission reactor for several reasons, including higher energy release and less radioactive waste production, the availability of fuel from seawater is not accurate.
- Fusion reactions require isotopes of hydrogen, such as deuterium and tritium, as fuel. Deuterium can be extracted from seawater, but tritium is a radioactive isotope that is not naturally abundant and needs to be produced artificially.
- Tritium can be produced by exposing lithium to neutron radiation, which can be generated by a fission reactor or a fusion reactor itself. However, the process of producing tritium is not as straightforward as extracting deuterium from seawater.
- Tritium is also highly radioactive and has a short half-life, which means it requires careful handling and containment. It cannot be easily stored or transported.
- Therefore, the fuel required for fusion reactions is not readily available in abundance from seawater, as stated in option B.
Correct statements:
a) For the same mass of substances involved, a fusion reaction releases much more energy than a fission reaction.
- This is true. Fusion reactions release a tremendous amount of energy, several times more than fission reactions. The fusion of hydrogen atoms into helium is the same process occurring in the Sun and other stars, which produces immense amounts of energy.
c) A fusion reaction can be much more easily controlled than a fission reaction.
- This is true. Fusion reactions require extremely high temperatures and pressures to sustain, and if these conditions are not maintained, the reaction will cease. This inherent stability makes fusion reactions more easily controllable than fission reactions, which can lead to runaway chain reactions if not properly regulated.
d) A fusion reaction produces almost no radioactive waste.
- This is true. Fusion reactions do not produce long-lived radioactive waste like fission reactions. The only radioactive byproduct of fusion is tritium, which has a relatively short half-life and can be managed safely.
In summary, option B is the wrong statement because the fuel required for fusion reactions is not readily available in abundance from seawater.
In a gene sanctuary genetic diversity of an endangered species can be preserved by :- a)protecting the ecosystem in which that species occurs naturally
- b)preserving DNA samples of different varieties in laboratory and growing them under controlled conditions.
- c)both A & B
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
In a gene sanctuary genetic diversity of an endangered species can be preserved by :
a)
protecting the ecosystem in which that species occurs naturally
b)
preserving DNA samples of different varieties in laboratory and growing them under controlled conditions.
c)
both A & B
d)
none of these

|
Krish Patel answered |
Conservation of genetic diversity under natural habitat of endangered species is called gene sanctuary.
The disintegration energy is- a)the difference between the initial energy and the total energy of the decay products
- b)the difference between the initial mass and the total mass of the decay products
- c)the difference between the initial mass energy and the total mass energy of the decay products
- d)none of the above
Correct answer is option 'C'. Can you explain this answer?
The disintegration energy is
a)
the difference between the initial energy and the total energy of the decay products
b)
the difference between the initial mass and the total mass of the decay products
c)
the difference between the initial mass energy and the total mass energy of the decay products
d)
none of the above
|
|
Rakibul Halsana answered |
CCCC
Which of the following particles can be added to the nucleus of an atom without changing its chemical properties?- a)Alpha Particles
- b)Protons
- c)Neutrons
- d)Electrons
Correct answer is option 'C'. Can you explain this answer?
Which of the following particles can be added to the nucleus of an atom without changing its chemical properties?
a)
Alpha Particles
b)
Protons
c)
Neutrons
d)
Electrons
|
|
Rajat Kapoor answered |
Adding a neutron to the nucleus will make no change in the chemical properties of the atom. The atom will have the same number of protons and therefore the same number of electrons. It is the number of electrons that determines chemical properties.
Actually, with hydrogen the addition of a neutron will almost double its mass and thus cause it to behave a little differently chemically.
Of course if the added neutron causes the nucleus to fission, decay, or otherwise change, that will change the chemistry.
When a hydrogen bomb explodes, which of the following is used?- a)fission
- b)both
- c)neither of two
- d)fusion
Correct answer is option 'B'. Can you explain this answer?
When a hydrogen bomb explodes, which of the following is used?
a)
fission
b)
both
c)
neither of two
d)
fusion
|
|
Naina Bansal answered |
Hydrogen bomb or H-bomb, weapon deriving a large portion of its energy from the nuclear fusion of hydrogen isotopes. In an atomic bomb, uranium or plutonium is split into lighter elements that together weigh less than the original atoms, the remainder of the mass appearing as energy. Unlike this fission bomb, the hydrogen bomb functions by the fusion, or joining together, of lighter elements into heavier elements. The end product again weighs less than its components, the difference once more appearing as energy. Because extremely high temperatures are required in order to initiate fusion reactions, the hydrogen bomb is also known as a thermonuclear bomb.
Alexander Von Humbolt described for the first time - a)Species area relationships
- b)Population Growth equation
- c)Ecological Biodiversity
- d)Laws of limiting factor
Correct answer is option 'A'. Can you explain this answer?
Alexander Von Humbolt described for the first time
a)
Species area relationships
b)
Population Growth equation
c)
Ecological Biodiversity
d)
Laws of limiting factor
|
|
Hitakshi answered |
Species area relationship is the relationship between
the area and the particular habitat. It was first studied by Alexander Von Humbolt. He observed that within a region species richness increased with increasing explored area, but only up to a limit. It is dependent on immigration, extinction and clustering etc. So, the correct answer is option 'A'.
the area and the particular habitat. It was first studied by Alexander Von Humbolt. He observed that within a region species richness increased with increasing explored area, but only up to a limit. It is dependent on immigration, extinction and clustering etc. So, the correct answer is option 'A'.
β -rays and γ-rays are respectively- a)neutrons and electromagnetic radiation of wavelengths shorter than X-rays
- b)protons and neutrons of wavelengths shorter than X-rays
- c)protons and electromagnetic radiation of wavelengths shorter than X-rays
- d)electrons and electromagnetic radiation of wavelengths shorter than X-rays
Correct answer is option 'D'. Can you explain this answer?
β -rays and γ-rays are respectively
a)
neutrons and electromagnetic radiation of wavelengths shorter than X-rays
b)
protons and neutrons of wavelengths shorter than X-rays
c)
protons and electromagnetic radiation of wavelengths shorter than X-rays
d)
electrons and electromagnetic radiation of wavelengths shorter than X-rays
|
|
Divyansh Kulkarni answered |
Beta radiation ~ Stream of electrons (unit negative charge). Beta positive radiation is when a positron is emitted rather than an electron.
Gamma radiation ~ Electromagnetic radiation of very short wavelength = high photon energy.
Gamma radiation ~ Electromagnetic radiation of very short wavelength = high photon energy.
A free neutron decays into- a)a proton, a positron and a antineutrino
- b)a proton, a positron and a neutrino
- c)a proton, an electron and an antineutrino
- d)a proton, an electron and a neutrino
Correct answer is option 'C'. Can you explain this answer?
A free neutron decays into
a)
a proton, a positron and a antineutrino
b)
a proton, a positron and a neutrino
c)
a proton, an electron and an antineutrino
d)
a proton, an electron and a neutrino
|
|
Rajeev Saxena answered |
The decay of free neutrons is energy feasible because the mass of a neutron is greater than the sum of the masses of the proton and electron it decays into. But where a neutron is paired with a proton its decay is not energy feasible and thus such neutrons within nuclei are stable.
How many species in the world are facing threat of extinction?- a)More than 50,500.
- b)Less than 1,550
- c)More than 15,500.
- d)No more species are endangered.
Correct answer is option 'C'. Can you explain this answer?
How many species in the world are facing threat of extinction?
a)
More than 50,500.
b)
Less than 1,550
c)
More than 15,500.
d)
No more species are endangered.

|
Ayush Choudhury answered |
More than 15,500 species worldwide is facing threat of extinction which includes amphibians, gymnosperms, reptiles, birds and mammals etc.
Rare species are :
i. represented by small populations in the world.
ii. thinly distributed over a wide area.
iii. all are grouped as endangered or vulnerable at present. - a)only i & ii are correct.
- b)only i & iii are correct.
- c)only ii & iii are correct.
- d)all are correct.
Correct answer is option 'A'. Can you explain this answer?
Rare species are :
i. represented by small populations in the world.
ii. thinly distributed over a wide area.
iii. all are grouped as endangered or vulnerable at present.
i. represented by small populations in the world.
ii. thinly distributed over a wide area.
iii. all are grouped as endangered or vulnerable at present.
a)
only i & ii are correct.
b)
only i & iii are correct.
c)
only ii & iii are correct.
d)
all are correct.

|
Shruti Chauhan answered |
Rare species are represented by small populations in the world. They are thinly distributed over wide area. Hence both statements are correct.
How many species of plants contribute to the traditional medicines used by native peoples around the world?- a)2,500
- b)2,000
- c)25,000
- d)5,000
Correct answer is option 'C'. Can you explain this answer?
How many species of plants contribute to the traditional medicines used by native peoples around the world?
a)
2,500
b)
2,000
c)
25,000
d)
5,000

|
EduRev NEET answered |
- The species of plants contribute to the traditional medicines used by native peoples around the world are approximately 25,000.
- Some examples of traditional plants used for medicinal purposes are ginger, garlic, chamomile, tulsi, etc.
To preserve seeds that rapidly lose viability, can’t survive dessication and plants which are propagated vegetatively, method employed is :- a)cryopreservation
- b)agroforestry
- c)gene sanctuary
- d)in - situ conservation
Correct answer is option 'A'. Can you explain this answer?
To preserve seeds that rapidly lose viability, can’t survive dessication and plants which are propagated vegetatively, method employed is :
a)
cryopreservation
b)
agroforestry
c)
gene sanctuary
d)
in - situ conservation

|
Ayush Choudhury answered |
Cryopreservation is the method of preserving living organisms or their parts at extremely low temperature. To preserve seeds that rapidly lose viability and cannot survive desiccation are preserved by this method.
The reaction of primary amine with Chloroform and ethanoic solution of KOH is called:- a)Hoffmann’s reaction
- b)Reimer Tiemann’s reaction
- c)Kolbe’s reaction
- d)Carbylamine reaction
Correct answer is option 'D'. Can you explain this answer?
The reaction of primary amine with Chloroform and ethanoic solution of KOH is called:
a)
Hoffmann’s reaction
b)
Reimer Tiemann’s reaction
c)
Kolbe’s reaction
d)
Carbylamine reaction
|
|
Naina Bansal answered |
The carbylamine reaction, also known as Hoffman's isocyanide test is a chemical test for detection of primary amines. In this reaction, the analyte is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide (carbylamine) is formed which are foul smelling substances.
When a hydrogen bomb explodes, which of the following is used?- a)fission
- b)fusion
- c)neither of two
- d)both
Correct answer is option 'D'. Can you explain this answer?
When a hydrogen bomb explodes, which of the following is used?
a)
fission
b)
fusion
c)
neither of two
d)
both

|
Dr Manju Sen answered |
The hydrogen bomb is a nuclear weapon that uses a mixture of fission and fusion to produce a massive explosion.
The nuclear fission generates enough heat to initiate the nuclear fusion reaction. After that, the nuclear fusion releases enormous amounts of energy, making the hydrogen bomb a lot more powerful than an atomic bomb.
The nuclear fission generates enough heat to initiate the nuclear fusion reaction. After that, the nuclear fusion releases enormous amounts of energy, making the hydrogen bomb a lot more powerful than an atomic bomb.
Nuclear forces are- a)spin dependent and have no non-central part
- b)spin dependent and have a non-central part
- c)spin independent and have no non-central part
- d)spin independent and have a non-central part
Correct answer is option 'D'. Can you explain this answer?
Nuclear forces are
a)
spin dependent and have no non-central part
b)
spin dependent and have a non-central part
c)
spin independent and have no non-central part
d)
spin independent and have a non-central part
|
|
Samridhi Yadav answered |
Nuclear Forces Overview
Nuclear forces, primarily responsible for holding the nucleus of an atom together, exhibit specific characteristics that distinguish them from other fundamental forces.
Spin Independence
- Nuclear forces are largely spin independent. This means that the strength and nature of the interaction do not depend on the intrinsic angular momentum (spin) of the nucleons (protons and neutrons).
Non-Central Forces
- These forces have a non-central part. In contrast to central forces, which act along the line connecting two particles, non-central forces can depend on the relative orientations of the spins of the nucleons. This non-centrality means that these forces can vary based on the spatial configuration and angular momentum of the interacting nucleons.
Implications of Spin Independence and Non-Centrality
- The spin independence of nuclear forces implies that nucleons can interact regardless of their spin states. This is crucial for the stability of atomic nuclei, as it allows for various configurations of nucleons.
- The non-central aspect allows for additional complexities in nuclear interactions, contributing to phenomena such as nuclear deformation and the formation of different nuclear states.
Conclusion
- In summary, the correct characterization of nuclear forces is that they are spin independent and possess a non-central part. This understanding is essential for grasping the underlying principles of nuclear physics and the behavior of atomic nuclei.
Nuclear forces, primarily responsible for holding the nucleus of an atom together, exhibit specific characteristics that distinguish them from other fundamental forces.
Spin Independence
- Nuclear forces are largely spin independent. This means that the strength and nature of the interaction do not depend on the intrinsic angular momentum (spin) of the nucleons (protons and neutrons).
Non-Central Forces
- These forces have a non-central part. In contrast to central forces, which act along the line connecting two particles, non-central forces can depend on the relative orientations of the spins of the nucleons. This non-centrality means that these forces can vary based on the spatial configuration and angular momentum of the interacting nucleons.
Implications of Spin Independence and Non-Centrality
- The spin independence of nuclear forces implies that nucleons can interact regardless of their spin states. This is crucial for the stability of atomic nuclei, as it allows for various configurations of nucleons.
- The non-central aspect allows for additional complexities in nuclear interactions, contributing to phenomena such as nuclear deformation and the formation of different nuclear states.
Conclusion
- In summary, the correct characterization of nuclear forces is that they are spin independent and possess a non-central part. This understanding is essential for grasping the underlying principles of nuclear physics and the behavior of atomic nuclei.
Chapter doubts & questions for February Week 2 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of February Week 2 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup