All questions of Temperature, Heat, and Thermal Energy for Grade 9 Exam
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:- a)2°C
- b)0°C
- c)5°C
- d)2.5°C
Correct answer is option 'B'. Can you explain this answer?
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:
a)
2°C
b)
0°C
c)
5°C
d)
2.5°C
|
|
Riya Banerjee answered |
Heat absorbed by 5g ice when it converted to at 0° C = 5 x 80 = 400 cal.
Heat liberated by 10g water at 10° C to 0° C = 100 cal
Hence there is 15g water at 0° C and 300 cal needs to be liberated , thus for some amount of water converts into ice, hence the temp of mixture is 0° C.
Heat liberated by 10g water at 10° C to 0° C = 100 cal
Hence there is 15g water at 0° C and 300 cal needs to be liberated , thus for some amount of water converts into ice, hence the temp of mixture is 0° C.
A steel tape is calibrated at 20° C. A piece of wood is being measured by steel tape at 10°C and reading is 30 cm on the tape. The real length of the wood is:
a)Equal to 30 cmb)less than 30 cmc)cannot be perdictedd)more than 30 cmCorrect answer is option 'B'. Can you explain this answer?
|
|
Preeti Khanna answered |
When heated the length between adjacent markings increases. Hence the actual length will be less than
30 cm.
30 cm.
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)- a)5R/2
- b)3 R
- c)2R
- d)5 R
Correct answer is option 'D'. Can you explain this answer?
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)
a)
5R/2
b)
3 R
c)
2R
d)
5 R
|
|
Hansa Sharma answered |
At constant pressure
dQ= nCpdT
=1*(5R/2)*2
=5R
dQ= nCpdT
=1*(5R/2)*2
=5R
Can you explain the answer of this question below:Water is used as coolant in automobiles radiators because
- A:
it has high specific heat capacity
- B:
it is easily available
- C:
it is easy to carry
- D:
it is cheap
The answer is a.
Water is used as coolant in automobiles radiators because
it has high specific heat capacity
it is easily available
it is easy to carry
it is cheap
|
|
Lavanya Menon answered |
Water is used as a coolant in automobiles radiators because it has high specific heat capacity. So, it absorbs a large amount of heat for a degree rise in temperature.
The temperature range upto which Newton’s law of cooling holds good- a)30-35°C
- b)25-30°C
- c)30-40°C
- d)20-25°C
Correct answer is option 'D'. Can you explain this answer?
The temperature range upto which Newton’s law of cooling holds good
a)
30-35°C
b)
25-30°C
c)
30-40°C
d)
20-25°C
|
|
Hansa Sharma answered |
Newton’s law of cooling is to be used at temperatures around room temperature.
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is- a)(C1t1 + C2t2 + C3t3)/3(C1 + C2 + C1)
- b)(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
- c)(t1 + t2 +t3 )/3
- d)3(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
Correct answer is option 'B'. Can you explain this answer?
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is
a)
(C1t1 + C2t2 + C3t3)/3(C1 + C2 + C1)
b)
(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
c)
(t1 + t2 +t3 )/3
d)
3(C1t1 + C2t2 + C3t3)/(C1 + C2 + C1)
|
|
Raghav Bansal answered |
For the composite system, energy conservation yields no net energy flow in or out of the system.
Let final temperature be T
Then, heat absorbed by A+heat absorbed by B+heat absorbed by C=0
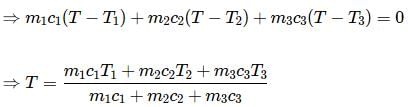
Here in the question m1=m2=m3
Let final temperature be T
Then, heat absorbed by A+heat absorbed by B+heat absorbed by C=0
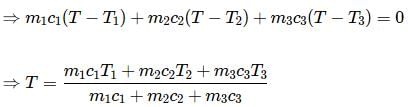
Here in the question m1=m2=m3
When water is heated from 0° C to 20° C its volume:- a)first decreases and then increases
- b)goes on increasing
- c)remains constant up to 15°C and then increases
- d)goes on decreasing
Correct answer is option 'A'. Can you explain this answer?
When water is heated from 0° C to 20° C its volume:
a)
first decreases and then increases
b)
goes on increasing
c)
remains constant up to 15°C and then increases
d)
goes on decreasing
|
|
Pooja Shah answered |
When water is heated from 0°C, its volume decreases because its density increases and you can see this effect upto 4°C. Since the density of ice is maximum at 4°C, afterwards as the density decreases the volume increases. The main reason for this is hydrogen bond in ice gets cleaved due to the melting of ice.
The heat of sun comes to us through:- a)convection
- b)conduction
- c)Sublimation
- d)Radiation
Correct answer is option 'D'. Can you explain this answer?
The heat of sun comes to us through:
a)
convection
b)
conduction
c)
Sublimation
d)
Radiation
|
|
Om Desai answered |
The sun heats the earth through radiation. Since there is no medium (like the gas in our atmosphere) in space, radiation is the primary way that heat travels in space. When the heat reaches the earth it warms the molecules of the atmosphere, and they warm other molecules and so on.
Water contract on heating between the temperatures- a)0°C to 4°C
- b)0°K to 4°K
- c)0o K to 273 K
- d)0°F to 4°F
Correct answer is option 'A'. Can you explain this answer?
Water contract on heating between the temperatures
a)
0°C to 4°C
b)
0°K to 4°K
c)
0o K to 273 K
d)
0°F to 4°F
|
|
Geetika Shah answered |
When water is heated from 0oC, its volume decreases because its density increases and you can see this effect upto 4oC. Since the density of ice is maximum at 4oC, afterwards as the density decreases the volume increases. The main reason for this is hydrogen bond in ice gets cleaved due to the melting of ice.
The process by which heat is transferred from one place to another without heating the intervening medium is known as- a)convection
- b)radiation
- c)sublimation
- d)fusion
Correct answer is option 'B'. Can you explain this answer?
The process by which heat is transferred from one place to another without heating the intervening medium is known as
a)
convection
b)
radiation
c)
sublimation
d)
fusion

|
Pioneer Academy answered |
Radiation alludes to the mechanism in which heat is transmitted without any physical contact between objects. It uses electromagnetic waves for transfer of heat.
A piece of iron of mass 100g is kept inside a furnace for a long time and Jthen put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.- a)500°C
- b)900°C
- c)953.6∘C
- d)706.80 °C
Correct answer is option 'C'. Can you explain this answer?
A piece of iron of mass 100g is kept inside a furnace for a long time and Jthen put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.
a)
500°C
b)
900°C
c)
953.6∘C
d)
706.80 °C
|
|
Gaurav Kumar answered |
Mass of Iron = 100g
Water Eq of caloriemeter = 10g
Mass of water = 240g
Let the Temp. of surface = 0ºC
Siron = 470J/kg°C
Water Eq of caloriemeter = 10g
Mass of water = 240g
Let the Temp. of surface = 0ºC
Siron = 470J/kg°C
Total heat gained = Total heat lost.
So,100/1000× 470 × (θ – 60) = 250/1000 × 4200 × (60 – 20)
⇒ 47θ – 47 × 60 = 25 × 42 × 40
⇒ θ = 4200 + 2820/47= 44820/47 =953.61°C
⇒ 47θ – 47 × 60 = 25 × 42 × 40
⇒ θ = 4200 + 2820/47= 44820/47 =953.61°C
What is the expression for temperature gradient?- a)
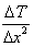
- b)
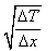
- c)
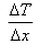
- d)
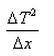
Correct answer is option 'C'. Can you explain this answer?
What is the expression for temperature gradient?
a)
b)
c)
d)
|
|
Krishna Iyer answered |
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location.
The temperature gradient is a dimensional quantity expressed in units of degrees per unit length.
The SI unit is kelvin per meter.
Expression: ∆T/∆x, where ∆T = change in temperature and ∆x = change in distance
The temperature gradient is a dimensional quantity expressed in units of degrees per unit length.
The SI unit is kelvin per meter.
Expression: ∆T/∆x, where ∆T = change in temperature and ∆x = change in distance
According to law of calorimetry, which of the given relation is true?- a)Heat gained ≥ Heat lost
- b)Heat gained = Heat lost
- c)Heat gained > Heat lost
- d)Heat lost > Heat gained
Correct answer is option 'B'. Can you explain this answer?
According to law of calorimetry, which of the given relation is true?
a)
Heat gained ≥ Heat lost
b)
Heat gained = Heat lost
c)
Heat gained > Heat lost
d)
Heat lost > Heat gained
|
|
Anjali Iyer answered |
A principle of calorimetry states that if there is no loss of heat in surrounding the total heat lost by hot body equal to the total heat gained by a cold body.
i.e. heat loss = heat gain
The temperature for which the reading on Celsius and Fahrenheit scales are identical is- a)-273°C, -273 °F
- b)-30°C, -30 °F
- c)0 °C, 0 °F
- d)-40 °C, -40 °F
Correct answer is option 'D'. Can you explain this answer?
The temperature for which the reading on Celsius and Fahrenheit scales are identical is
a)
-273°C, -273 °F
b)
-30°C, -30 °F
c)
0 °C, 0 °F
d)
-40 °C, -40 °F
|
|
Preeti Iyer answered |
The Celsius and Fahrenheit are two important temperature scales. The Fahrenheit scale is used primarily in the United States, while Celsius is used throughout the world. The two scales have different zero points and the Celsius degree is bigger than the Fahrenheit one. There is one point on the Fahrenheit and Celsius scales where the temperatures in degrees are equal. This is -40 degree C and -40 degree F.
A device in which heat measurement can be made is called- a)Joule meter
- b)Calorimeter
- c)Thermal meter
- d)Gauge meter
Correct answer is option 'B'. Can you explain this answer?
A device in which heat measurement can be made is called
a)
Joule meter
b)
Calorimeter
c)
Thermal meter
d)
Gauge meter

|
Shanaya Tiwari answered |
Calorimeter:
The correct device for heat measurement is a calorimeter. Calorimeters are used to measure the heat involved in a chemical reaction or physical change. They work based on the principle of conservation of energy, where the heat released or absorbed during a reaction is measured by the change in temperature of a known mass of water.
How does a Calorimeter work?
- A known quantity of the substance undergoing the reaction is placed in the calorimeter along with a known quantity of water.
- The initial temperature of the water and the substance is recorded.
- The reaction takes place, and the heat released or absorbed causes a change in temperature of the water.
- By measuring the change in temperature, the heat exchanged can be calculated using the specific heat capacity of water.
- Calorimeters are designed to minimize heat loss to the surroundings to ensure accurate measurements.
Types of Calorimeters:
- There are different types of calorimeters such as bomb calorimeters, coffee cup calorimeters, and differential scanning calorimeters, each suitable for different applications.
- Bomb calorimeters are commonly used for measuring the heat of combustion of substances.
- Coffee cup calorimeters are simpler and are often used in educational settings to demonstrate heat exchange principles.
In conclusion, a calorimeter is the appropriate device for heat measurement as it allows for accurate determination of the heat involved in a reaction or process.
The correct device for heat measurement is a calorimeter. Calorimeters are used to measure the heat involved in a chemical reaction or physical change. They work based on the principle of conservation of energy, where the heat released or absorbed during a reaction is measured by the change in temperature of a known mass of water.
How does a Calorimeter work?
- A known quantity of the substance undergoing the reaction is placed in the calorimeter along with a known quantity of water.
- The initial temperature of the water and the substance is recorded.
- The reaction takes place, and the heat released or absorbed causes a change in temperature of the water.
- By measuring the change in temperature, the heat exchanged can be calculated using the specific heat capacity of water.
- Calorimeters are designed to minimize heat loss to the surroundings to ensure accurate measurements.
Types of Calorimeters:
- There are different types of calorimeters such as bomb calorimeters, coffee cup calorimeters, and differential scanning calorimeters, each suitable for different applications.
- Bomb calorimeters are commonly used for measuring the heat of combustion of substances.
- Coffee cup calorimeters are simpler and are often used in educational settings to demonstrate heat exchange principles.
In conclusion, a calorimeter is the appropriate device for heat measurement as it allows for accurate determination of the heat involved in a reaction or process.
Newton’s law of cooling states that the rate of cooling of a body is proportional to the _____________________.- a)temperature of the surroundings
- b)excess temperature of the body over the surroundings
- c)temperature of the body
- d)temperature of the body + temperature of the surroundings
Correct answer is option 'B'. Can you explain this answer?
Newton’s law of cooling states that the rate of cooling of a body is proportional to the _____________________.
a)
temperature of the surroundings
b)
excess temperature of the body over the surroundings
c)
temperature of the body
d)
temperature of the body + temperature of the surroundings
|
|
Geetika Shah answered |
Newton's law of cooling states that the heat released by a body with respect to time (or) the rate of heat released is directly proportional to the difference between the body's temperature and the surrounding temperature.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
Can you explain the answer of this question below:Two identical rectangular strips, one of copper and the other of steel, are riveted as shown to form a bi-metal strip. On heating, the bi-metal strip will

- A:
bend with steel on the concave side
- B:
get twisted
- C:
remain straight
- D:
bend with steel on the convex side
The answer is a.
Two identical rectangular strips, one of copper and the other of steel, are riveted as shown to form a bi-metal strip. On heating, the bi-metal strip will

bend with steel on the concave side
get twisted
remain straight
bend with steel on the convex side
|
|
Nandini Patel answered |
On heating, the copper strip will suffer greater elongation and hence the bimetal strip will bend with the steel strip on the concave side.
[Bimetal strips are widely used in thermal switching applications such as automatic electric iron].
What should be the temperature of black body to emit radiant energy which is independent of the conditions in the surroundings?- a)temperature of black body should be less than zero
- b)temperature of black body should be more than zero
- c)temperature of black body should be equal to zero
- d)all of the above
Correct answer is option 'B'. Can you explain this answer?
What should be the temperature of black body to emit radiant energy which is independent of the conditions in the surroundings?
a)
temperature of black body should be less than zero
b)
temperature of black body should be more than zero
c)
temperature of black body should be equal to zero
d)
all of the above
|
|
Suresh Reddy answered |
Temperature of black body should be more than zero. Obvious answer. If the temperature is not greater than 0 then there will be no radiation.
What is the coefficient of thermal conductivity K?- a)ΔQt
- b)
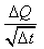
- c)
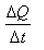
- d)
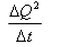
Correct answer is option 'C'. Can you explain this answer?
What is the coefficient of thermal conductivity K?
a)
ΔQt
b)
c)
d)

|
Ciel Knowledge answered |
K is rate of change of charge.
Mercury is a liquid which is heated by:- a)Convection
- b)Radiation
- c)Conduction
- d)transfusion
Correct answer is option 'C'. Can you explain this answer?
Mercury is a liquid which is heated by:
a)
Convection
b)
Radiation
c)
Conduction
d)
transfusion
|
|
Geetika Shah answered |
Hg expands on heat (thermometers) it acts as a good conducting metal.
Which phase of matter has maximum value of temperature coefficient of cubical expansion?- a)Gaseous
- b)Solid
- c)Liquid
- d)none of these
Correct answer is option 'A'. Can you explain this answer?
Which phase of matter has maximum value of temperature coefficient of cubical expansion?
a)
Gaseous
b)
Solid
c)
Liquid
d)
none of these
|
|
Pooja Shah answered |
Gas expands more than solid and liquid because gas particles are far apart hence freely move.
Value of coefficient of thermal coefficient is:- a)same incase of conductors and insulators
- b)Good incase of conductors and small incase of insulators
- c)Does not depend on insulators and conductors
- d)Small incase of insulators and good incase of insulators
Correct answer is option 'B'. Can you explain this answer?
Value of coefficient of thermal coefficient is:
a)
same incase of conductors and insulators
b)
Good incase of conductors and small incase of insulators
c)
Does not depend on insulators and conductors
d)
Small incase of insulators and good incase of insulators
|
|
Raghav Bansal answered |
Conductors have free electrons in them whereas insulators don’t have any. Therefore, conductors conduct heat and electricity better than insulators. Therefore the value of thermal coefficient is good in case of conductors and less in case in insulators.
Which of the given phenomenon is not related to convection?- a)In winter metallic handles appear colder than wooden door
- b)Maintaining comfortable room temperature in cold countries
- c)Formation of trade winds
- d)Formation of land and sea breezes
Correct answer is option 'A'. Can you explain this answer?
Which of the given phenomenon is not related to convection?
a)
In winter metallic handles appear colder than wooden door
b)
Maintaining comfortable room temperature in cold countries
c)
Formation of trade winds
d)
Formation of land and sea breezes

|
Harika Chittiboyina answered |
Convection is the movement of surrounding medium according to density by which transfer of heat takes place. Whereas in option A it tells about the transfer of heat due to molecules inside conductor which is conduction
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as- a)conduction
- b)convection
- c)Sublimation
- d)Fusion
Correct answer is option 'A'. Can you explain this answer?
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as
a)
conduction
b)
convection
c)
Sublimation
d)
Fusion
|
|
Geetika Shah answered |
The mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference is known as conduction. This is the definition of conduction.
The process by which heat flows from the region of higher temperature to the region of lower temperature by actual movement of material particles is called- a)Sublimation
- b)radiaton
- c)Conduction
- d)Convection
Correct answer is option 'D'. Can you explain this answer?
The process by which heat flows from the region of higher temperature to the region of lower temperature by actual movement of material particles is called
a)
Sublimation
b)
radiaton
c)
Conduction
d)
Convection
|
|
Rajat Patel answered |
In this process, heat is transferred in the liquid and gases from a region of higher temperature to a region of lower temperature. Convection heat transfer occurs partly due to the actual movement of molecules or due to the mass transfer.
SI unit of thermal conductivity is- a)Js K
- b)Js-1 K
- c)Js K-1
- d)Jm-1s-1 K-1
Correct answer is option 'D'. Can you explain this answer?
SI unit of thermal conductivity is
a)
Js K
b)
Js-1 K
c)
Js K-1
d)
Jm-1s-1 K-1
|
|
Pooja Shah answered |
K = power × thickness (m) ÷ area × ∆T
K = (J s-1) (m) ÷ (m2) (K)
K = J m-1 s-1 k-1
K = (J s-1) (m) ÷ (m2) (K)
K = J m-1 s-1 k-1
If the temperature difference between the body and surrounding is small, then the rate of loss of heat is directly proportional to- a)Temperature of two bodies
- b)Nature of two bodies
- c)Pressure difference of the body
- d)Size of the two bodies
Correct answer is option 'A'. Can you explain this answer?
If the temperature difference between the body and surrounding is small, then the rate of loss of heat is directly proportional to
a)
Temperature of two bodies
b)
Nature of two bodies
c)
Pressure difference of the body
d)
Size of the two bodies
|
|
Hansa Sharma answered |
The rate of loss of heat by a body is directly proportional to its excess temperature over that of the surroundings provided that this excess is small.
Among the following methods of heat transfer, gravity does not play any part in- a)Convection
- b)Radiation and conduction
- c)radiation
- d)convection and conduction
Correct answer is option 'B'. Can you explain this answer?
Among the following methods of heat transfer, gravity does not play any part in
a)
Convection
b)
Radiation and conduction
c)
radiation
d)
convection and conduction
|
|
Rajat Patel answered |
Gravity does not play any part in radiation and conduction because in both these processes heat is transferred without any motion of the medium particles.
The relation between coefficient of volume expansion 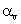 and coefficient of linear expansion
and coefficient of linear expansion 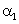 is
is- a)αv = 2α1
- b)α1 = 3αv
- c)αv = 3α1
- d)α1 = 2αv
Correct answer is option 'C'. Can you explain this answer?
The relation between coefficient of volume expansion 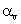 and coefficient of linear expansion
and coefficient of linear expansion 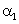 is
is
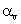 and coefficient of linear expansion
and coefficient of linear expansion 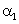 is
isa)
αv = 2α1
b)
α1 = 3αv
c)
αv = 3α1
d)
α1 = 2αv

|
The Amazing Spider Fan answered |
This is simple,because the coefficients of linear expansion A,areal expansion B,and volume expansion G,are in the ratio of 1:2:3 i.e;
A:B:G::1:2:3
Hence,G=3A
Four cylindrical rods of different radii and lengths are used to connect two heat reservoirs at fixed temperatures t1 and t2 respectively. From the following pick out the rod which will conduct the maximum quantity of heat:- a)Radius 1 cm, length 2 m
- b)Radius 1 cm, length 1 m
- c)Radius 2 cm, length 4 m
- d)Radius 3 cm, length 8 m
Correct answer is option 'D'. Can you explain this answer?
Four cylindrical rods of different radii and lengths are used to connect two heat reservoirs at fixed temperatures t1 and t2 respectively. From the following pick out the rod which will conduct the maximum quantity of heat:
a)
Radius 1 cm, length 2 m
b)
Radius 1 cm, length 1 m
c)
Radius 2 cm, length 4 m
d)
Radius 3 cm, length 8 m
|
|
Neha Sharma answered |
The rate of heat transfer is directly proportional to the bisectional surface area of the solid and inversely proportional to its parallel length.
That is heat conduction rate say H ∝ r2
∝ 1/L
I.e ∝ r2/L
Hence the conduction would be
maximum in case in which r2/L ratio is largest.
That is heat conduction rate say H ∝ r2
∝ 1/L
I.e ∝ r2/L
Hence the conduction would be
maximum in case in which r2/L ratio is largest.
The coefficient of liner expansion of a cubical crystal along three mutually perpendicular direction is  and
and  . What is the coefficient of cubical expansion of crystal?
. What is the coefficient of cubical expansion of crystal?- a)α1 + α2 + α3
- b)α1 + α2 - α3
- c)α1 - α2 - α3
- d)(α1 + α2 )/ α3
Correct answer is option 'A'. Can you explain this answer?
The coefficient of liner expansion of a cubical crystal along three mutually perpendicular direction is  and
and  . What is the coefficient of cubical expansion of crystal?
. What is the coefficient of cubical expansion of crystal?
 and
and  . What is the coefficient of cubical expansion of crystal?
. What is the coefficient of cubical expansion of crystal?a)
α1 + α2 + α3
b)
α1 + α2 - α3
c)
α1 - α2 - α3
d)
(α1 + α2 )/ α3
|
|
Neha Joshi answered |
Cubical expansion means there is expansion is length (a1), breadth (a2) and height (a3). Therefore the net coefficient of cubical expansion is α1 + α2 + α3.
Which is the fastest mode of heat transfer?- a)convection
- b)conduction
- c)Transition
- d)Radiation
Correct answer is option 'D'. Can you explain this answer?
Which is the fastest mode of heat transfer?
a)
convection
b)
conduction
c)
Transition
d)
Radiation
|
|
Arun Khanna answered |
Answer: Radiation is the fastest mode of transfer of heat, because radiation travels at the speed of light, which is very quick. The slowest mode of transfer of heat is conduction because it takes place from particle to particle.
What are units of K?- a)Wm-2K-1
- b)WmK
- c)Wm-1K-1
- d)Wm-1K-2
Correct answer is option 'C'. Can you explain this answer?
What are units of K?
a)
Wm-2K-1
b)
WmK
c)
Wm-1K-1
d)
Wm-1K-2

|
Rishika Mishra answered |
Understanding K
K, known as thermal conductivity, is a property of materials that indicates their ability to conduct heat. The units of K are crucial for understanding heat transfer.
Unit Analysis of Options
- Option A: Wm-2K-1
- This unit represents watts per square meter per Kelvin, typically used for heat transfer per unit area, not for thermal conductivity.
- Option B: WmK
- This unit suggests watts per meter Kelvin, which does not correctly express the relationship of heat transfer through a material.
- Option C: Wm-1K-1
- This is the correct unit for thermal conductivity. It represents watts per meter per Kelvin, indicating how much heat (in watts) can pass through a meter of the material for every degree of temperature difference (in Kelvin).
- Option D: Wm-1K-2
- This unit indicates watts per meter per Kelvin squared, which does not correspond to thermal conductivity and is not relevant in this context.
Conclusion
K = Wm-1K-1 signifies that for every meter of material, how effectively it conducts heat is measured against a temperature difference. Understanding this unit is essential for applications in engineering and physics, especially in thermal management and insulation design. Thus, option C is the correct answer for the units of K.
K, known as thermal conductivity, is a property of materials that indicates their ability to conduct heat. The units of K are crucial for understanding heat transfer.
Unit Analysis of Options
- Option A: Wm-2K-1
- This unit represents watts per square meter per Kelvin, typically used for heat transfer per unit area, not for thermal conductivity.
- Option B: WmK
- This unit suggests watts per meter Kelvin, which does not correctly express the relationship of heat transfer through a material.
- Option C: Wm-1K-1
- This is the correct unit for thermal conductivity. It represents watts per meter per Kelvin, indicating how much heat (in watts) can pass through a meter of the material for every degree of temperature difference (in Kelvin).
- Option D: Wm-1K-2
- This unit indicates watts per meter per Kelvin squared, which does not correspond to thermal conductivity and is not relevant in this context.
Conclusion
K = Wm-1K-1 signifies that for every meter of material, how effectively it conducts heat is measured against a temperature difference. Understanding this unit is essential for applications in engineering and physics, especially in thermal management and insulation design. Thus, option C is the correct answer for the units of K.
Which of the given relation is true for Newton’s law of cooling?- a)
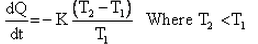
- b)
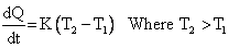
- c)
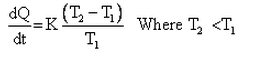
- d)
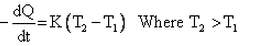
Correct answer is option 'D'. Can you explain this answer?
Which of the given relation is true for Newton’s law of cooling?
a)
b)
c)
d)
|
|
Arka Bose answered |
Newton's law of cooling states that the heat released by a body with respect to time (or) the rate of heat released is directly proportional to the difference between the body's temperature and the surrounding temperature.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
dH/dt = k(T – Ts) where t = surrounding's temperature and T = temperature of the body
Consider two bodies A and B, of equal surface areas, such that A's temperature is more that B's temperature and the surrounding temperature is less than both A and B. Then according to Newton's law of cooling A loses more heat to the surroundings when compared B during the same time interval. So, A will cool faster than B.
Chapter doubts & questions for Temperature, Heat, and Thermal Energy - Physics 2025 is part of Grade 9 exam preparation. The chapters have been prepared according to the Grade 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Grade 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Temperature, Heat, and Thermal Energy - Physics in English & Hindi are available as part of Grade 9 exam.
Download more important topics, notes, lectures and mock test series for Grade 9 Exam by signing up for free.
Physics
307 videos|482 docs|202 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup