All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Solubility (GC) for MCAT Exam
For HF, pKa = 3.45. What is the pH of an aqueous buffer solution that is 0.1M HF (aq) and 0.300 M KF (aq)?
a)11.03b)2.97c)10.07d)3.93 Correct answer is option 'D'. Can you explain this answer?
|
|
Suresh Iyer answered |
Given, pKa = 3.45
Concentration of HF = 0.1 M, concentration of KF = 0.300 M
For acidic buffer;
pH = pKa + log [salt of weak acid]/[weak acid]
= 3.45 + log0.3/0.1
= 3.45 + 0.48
= 3.93
Concentration of HF = 0.1 M, concentration of KF = 0.300 M
For acidic buffer;
pH = pKa + log [salt of weak acid]/[weak acid]
= 3.45 + log0.3/0.1
= 3.45 + 0.48
= 3.93
Consider KA, the potassium salt of a weak acid, HA. If a sample of KA is dissolved in water, with no other substances added, which of the following statements is true (approximately)?- a)[H3O+] = [OH-]
- b)[HA] = [K+]
- c)[HA] = [OH-]
- d)[H3O+] = [A-]
Correct answer is option 'C'. Can you explain this answer?
Consider KA, the potassium salt of a weak acid, HA. If a sample of KA is dissolved in water, with no other substances added, which of the following statements is true (approximately)?
a)
[H3O+] = [OH-]
b)
[HA] = [K+]
c)
[HA] = [OH-]
d)
[H3O+] = [A-]
|
|
Suresh Iyer answered |
The correct answer is option C
[HA] = [OH–]
The reaction is: A– + H2O ⇌ HA + OH–
[HA] = [OH–]
The reaction is: A– + H2O ⇌ HA + OH–
Role of NH4Cl in qualitative analysis of third group cations- a)to provide basic medium
- b)to provide acidic medium
- c)to increase the degree of dissociation of NH4OH
- d)to suppress the degree of dissociation of NH4OH
Correct answer is option 'D'. Can you explain this answer?
Role of NH4Cl in qualitative analysis of third group cations
a)
to provide basic medium
b)
to provide acidic medium
c)
to increase the degree of dissociation of NH4OH
d)
to suppress the degree of dissociation of NH4OH
|
|
Raghav Bansal answered |
The correct answer is option D
Common ion effect is observed when a solution of weak electrolyte is mixed with a solution of strong electrolyte, which provides an ion common to that provided by weak electrolyte.
The NH4OH is a weak base and it does not ionise completely. Thus, due to presence of common ion NH4+ in NH4Cl, it suppresses the ionisation of weak base NH4OH in order to decrease the OH- concentration so that higher group cations will not get precipitated.
Thus the pair NH4OH+NH4Cl shows a common ion effect.
Ammonium chloride suppresses the ionization of ammonium hydroxide
.
Common ion effect is observed when a solution of weak electrolyte is mixed with a solution of strong electrolyte, which provides an ion common to that provided by weak electrolyte.
The NH4OH is a weak base and it does not ionise completely. Thus, due to presence of common ion NH4+ in NH4Cl, it suppresses the ionisation of weak base NH4OH in order to decrease the OH- concentration so that higher group cations will not get precipitated.
Thus the pair NH4OH+NH4Cl shows a common ion effect.
Ammonium chloride suppresses the ionization of ammonium hydroxide
.
We eat a variety of foods still pH of our blood does not change every time. The reason is- a)Strong bases in the blood donot let pH change
- b)Stomach wall is resistant
- c)There are buffers in the blood which resist pH change
- d)Strong acids in the blood donot let pH change
Correct answer is option 'C'. Can you explain this answer?
We eat a variety of foods still pH of our blood does not change every time. The reason is
a)
Strong bases in the blood donot let pH change
b)
Stomach wall is resistant
c)
There are buffers in the blood which resist pH change
d)
Strong acids in the blood donot let pH change
|
|
Pooja Shah answered |
The correct answer is option C
pH of blood remains constant because of the buffer system present in the blood. Acid-base buffers confer resistance to a change in the pH of a solution when hydrogen ions (protons) or hydroxide ions are added or removed. An acid-base buffer typically consists of a weak acid, and its conjugate base (salt).It used to neutralized the extra added protons or OH- in blood.The buffer for maintaining acid-base balance in the blood is the carbonic-acid-bicarbonate buffer.So pH of blood remains even after eating spicy food.
pH of blood remains constant because of the buffer system present in the blood. Acid-base buffers confer resistance to a change in the pH of a solution when hydrogen ions (protons) or hydroxide ions are added or removed. An acid-base buffer typically consists of a weak acid, and its conjugate base (salt).It used to neutralized the extra added protons or OH- in blood.The buffer for maintaining acid-base balance in the blood is the carbonic-acid-bicarbonate buffer.So pH of blood remains even after eating spicy food.
Calcium fluoride (CaF2) has a Ksp value of 4.0 x 10−11. Which of the following statements correctly describes the solubility of CaF2 in a solution containing 0.10 M Ca(NO3)2 versus a solution containing 0.10 M NaF?- a)CaF2 is approximately 4 times more soluble in a solution containing 0.10 M Ca(NO3)2 than in a solution containing 0.10 M NaF.
- b)CaF2 is approximately 2500 times more soluble in a solution containing 0.10 M Ca(NO3)2 than in a solution containing 0.10 M NaF.
- c)The molar solubility of CaF2 in a solution containing 0.10 M NaF is 4.0 x 10−10 M.
- d)The molar solubility of CaF2 in a solution containing 0.10 M Ca(NO3)2 is 1 x 10−3 M.
Correct answer is option 'B'. Can you explain this answer?
Calcium fluoride (CaF2) has a Ksp value of 4.0 x 10−11. Which of the following statements correctly describes the solubility of CaF2 in a solution containing 0.10 M Ca(NO3)2 versus a solution containing 0.10 M NaF?
a)
CaF2 is approximately 4 times more soluble in a solution containing 0.10 M Ca(NO3)2 than in a solution containing 0.10 M NaF.
b)
CaF2 is approximately 2500 times more soluble in a solution containing 0.10 M Ca(NO3)2 than in a solution containing 0.10 M NaF.
c)
The molar solubility of CaF2 in a solution containing 0.10 M NaF is 4.0 x 10−10 M.
d)
The molar solubility of CaF2 in a solution containing 0.10 M Ca(NO3)2 is 1 x 10−3 M.

|
Orion Classes answered |
To calculate the molar solubility of CaF2 in a solution containing 0.10 M Ca(NO3)2, start with the Ksp equation and then replace the right side with actual concentration values:
4.0 x 10−11 = (Ca2+)(F⁻)2
4.0 x 10−11 = (0.1 + y)(2y)2
4.0 x 10−11 = (0.1 + y)(2y)2
When the existing concentration in the solution and the Ksp value differ by more than a power of 3, which means 103 or 1000, the equation can be simplified by approximating (0.1 + y) as 0.1. Ultimately the value of y would be 1000 times less compared to 0.10 M and can be ignored; for instance, 0.1003 or 0.1009 would round to 0.10.
After the approximation, isolate the variable y by dividing both sides by 0.4 and finding the square root of both sides by taking the square root of 1 and dividing the exponent by 2:
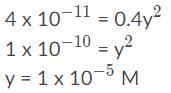
To calculate the molar solubility of CaF2 in a solution containing 0.10 M NaF, start with the equation:
4.0 x 10−11 = (y)(0.1 + 2y)2
Simplify the equation by approximating (0.1 + 2y) to be 0.1 and solve for y by dividing both sides of the equation by 0.01:
4.0 x 10−11 = (y)(0.1)2
y = 4.0 x 10−9 M
y = 4.0 x 10−9 M
Note the vast difference in solubility due to the common ion effect. 1.0 x 10−5 M and 4.0 x 10−9 M are the two molar solubilities, and they differ by a factor of about 2500. So CaF2 is approximately 2500 times more soluble in a solution containing 0.10 M Ca(NO3)2 than in a solution containing 0.10 M NaF.
If the value of the solubility product for AgBr is 4.0 x 10-12 at 25°C, calculate the solubility of AgBr(s) in water.- a)2.5 x 10-13
- b)2.5 x 10-6
- c)2 x 10-6
- d)5.0 x10-7
Correct answer is option 'C'. Can you explain this answer?
If the value of the solubility product for AgBr is 4.0 x 10-12 at 25°C, calculate the solubility of AgBr(s) in water.
a)
2.5 x 10-13
b)
2.5 x 10-6
c)
2 x 10-6
d)
5.0 x10-7
|
|
Raghav Bansal answered |
Ksp = 4.0 x 10-12
For AB type salt, Solubility = Ksp1/2
= (4.0 x 10-12)1/2
= 2 x 10-6
For AB type salt, Solubility = Ksp1/2
= (4.0 x 10-12)1/2
= 2 x 10-6
The following equilibrium exists in aqueous solutionCH3COOH 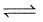 CH3COO- + H+
CH3COO- + H+
If dilute HCl is added:- a)Acetate ion concentration will increase
- b)Acetate ion concentration will decrease
- c)The equilibrium constant will decrease
- d)The equilibrium constant will increase.
Correct answer is option 'B'. Can you explain this answer?
The following equilibrium exists in aqueous solution
CH3COOH 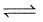 CH3COO- + H+
CH3COO- + H+
If dilute HCl is added:
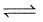 CH3COO- + H+
CH3COO- + H+If dilute HCl is added:
a)
Acetate ion concentration will increase
b)
Acetate ion concentration will decrease
c)
The equilibrium constant will decrease
d)
The equilibrium constant will increase.
|
|
Sahana Marigoudar answered |
H+ concentration increases which makes the reaction to proceed through reverse direction due to which acetic acid concentration increases.
Which of the following Lewis dot structures of polyatomic ions has or have an overall charge of -2?I. 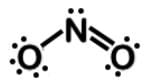
II. 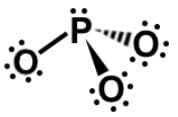
III. 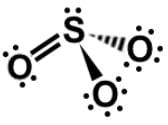
IV. 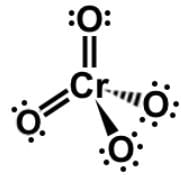
- a)I, III, and IV
- b)III and IV
- c)I, II, and IV
- d)III only
Correct answer is option 'B'. Can you explain this answer?
Which of the following Lewis dot structures of polyatomic ions has or have an overall charge of -2?
I. 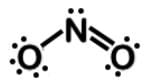
II.
III.
IV.
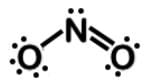
II.

III.

IV.

a)
I, III, and IV
b)
III and IV
c)
I, II, and IV
d)
III only
|
|
Ayesha Joshi answered |
It should be emphasized that these common polyatomic ions and their charges should be memorized for the MCAT. We may not be accustomed to looking at the Lewis dot structures for these polyatomic ions.
In analyzing the nitrite ion, NO2 when oxygen has 1 bond, it has a negative charge. When N has 3 and O has 2 bonds, they are uncharged, leaving nitrite ion with an overall charge of -1. It is worth noting that both nitrate (NO3) and nitrite (NO2), and have a charge of -1.
In analyzing the phosphite ion, PO3, since there are 3 single-bonded oxygen, they have a negative charge of -3. Phosphorus is in the same group as nitrogen, and so similarly, when P has 3 bonds, it is uncharged, leaving phosphite with an overall charge of -3. It is worth noting that both phosphate (PO4) and phosphite (PO3) have a charge of -3.
In analyzing the sulfite ion, SO3, we have an element that perpetually expands its octet. Sulfur has 6 valence electrons, so it can form up to 6 bonds. Since 4 are in single bonds and 2 in the lone pair, its formal charge is zero.
Applying the same logic, oxygen has 6 valence electrons. Since 1 is in the single bond and 6 are in the lone pairs, its formal charge is -1, leaving sulfite with an overall charge of -2. It is worth noting that both sulfate (SO4) and sulfite (SO3) have a charge of -2.
Lastly, chromate (CrO4) must be memorized separately with a charge of -2. Therefore, the correct answer is III and IV. For sake of thoroughness, all the oxyhalide anions, perchlorate (ClO4), chlorate (ClO3), chlorite (ClO2), and hypochlorite (ClO), all have a charge of -1.
Which of the following statements most accurately describes the data depicted in the graph depicting the concentration of silver ion at varying concentrations of chloride ion in a saturated solution of AgCl? (Ksp of Agcl = 1.8 x 10-10)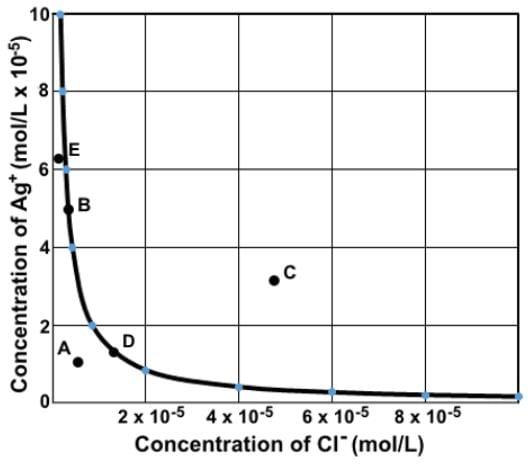
- a)Points B and D are both in equilibrium, but point B represents a case when there is a common ion effect.
- b)Point B has a greater concentration of ions dissolved than at point C. It would really help if this graph had a title and the solubility product of of AgCl were given.
- c)Point E has the greatest concentration of ions dissolved.
- d)Point D has a greater concentration of chloride ions dissolved than at point A, but both points have the same Ksp value.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements most accurately describes the data depicted in the graph depicting the concentration of silver ion at varying concentrations of chloride ion in a saturated solution of AgCl? (Ksp of Agcl = 1.8 x 10-10)

a)
Points B and D are both in equilibrium, but point B represents a case when there is a common ion effect.
b)
Point B has a greater concentration of ions dissolved than at point C. It would really help if this graph had a title and the solubility product of of AgCl were given.
c)
Point E has the greatest concentration of ions dissolved.
d)
Point D has a greater concentration of chloride ions dissolved than at point A, but both points have the same Ksp value.

|
Orion Classes answered |
This is a graph of the inverse relationship between Ag+ and Cl⁻, as expressed by the solubility product constant expression, Ksp = (Ag+)(Cl⁻).
All the points that make up the graph represents all the possible concentrations of Ag+ and Cl⁻ such that their product equals Ksp
Any points above or to the right of the graph would produce ion products of supersaturated solutions (Q > Ksp). Any points below or to the left of the graph would produce ion products of unsaturated solutions (Q < Ksp).
Point D does have a greater concentration of chloride ions dissolved than point A, but only point D has a Ksp value.
Point E does not have the greatest concentration of ions dissolved, but point C. It is the only point that is the supersaturated area.
Point B does not have a greater concentration of ions dissolved than at point C since point C is a supersaturated solution.
Points B and D are both in equilibrium since they lie on the graph. Point B could represent the case where there is an existing concentration of Ag+ in the solution. Point D represents the point where the two ion concentrations are equal.
Which of the following conditions will allow cupric hydroxide to dissolve in a saturated solution of Cu(OH)2?- a)Increasing the pH of the solution
- b)Adding copper phosphate to the solution
- c)Adding sodium hydroxide to the solution
- d)Adding ammonia to the solution
Correct answer is option 'D'. Can you explain this answer?
Which of the following conditions will allow cupric hydroxide to dissolve in a saturated solution of Cu(OH)2?
a)
Increasing the pH of the solution
b)
Adding copper phosphate to the solution
c)
Adding sodium hydroxide to the solution
d)
Adding ammonia to the solution
|
|
Ayesha Joshi answered |
Apply Le Châtelier’s principle to the below solubility equilibrium. Depending on the stress that is applied on the system, more may precipitate or dissolve.
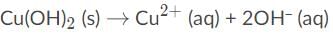
By adding NaOH to the solution, the concentration of hydroxide ion will increase. When the concentration of a product increases, the reaction shifts back to the left, and here, more Cu(OH)2 will precipitate.
By adding copper phosphate to the solution, there will be little effect on the equilibrium since Cu3(PO4)2 is an insoluble solid.
Increasing the pH is the same as increasing the basicity, which can be achieved by adding more hydroxide ion or removing hydronium ion. In either case, more Cu(OH)2 will not dissolve.
The goal of adding ammonia was not to increase the basicity of the solution, but act as a ligand that binds to a central metal atom to form a coordination complex. The halides and pseudohalides are important anionic ligands whereas ammonia, carbon monoxide, and water are common charge-neutral ligands.
By adding ammonia to the solution, copper forms a complex ion with ammonia to become the copper ammine complex [Cu(NH3)4]2+. This will remove Cu2+ from the solution, and more Cu(OH)2 will dissolve.
The correct answer is to add ammonia to the solution to allow more cupric hydroxide to dissolve.
Based on the solubility product constants below, what is the correct order of the following hydroxide solutions in increasing pH?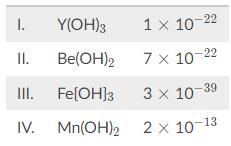
- a)III → I → II → IV
- b)III → lI → I → IV
- c)IV → I → II → III
- d)IV → II → I → III
Correct answer is option 'B'. Can you explain this answer?
Based on the solubility product constants below, what is the correct order of the following hydroxide solutions in increasing pH?

a)
III → I → II → IV
b)
III → lI → I → IV
c)
IV → I → II → III
d)
IV → II → I → III

|
Orion Classes answered |
The temptation is to use the solubility product constants (Ksp) as a direct measure of concentration, but the proper method is to compare the molar solubilities. Ksp values can be used when all the compounds dissociate into the same number of ions, but since the solids here dissociate into a different number of ions, the molar solubilities should be calculated.
Here is a shortcut to determine the rank without much math. Divide the exponent by the number of ions to give you a rough estimate of the magnitude of the negative exponent. For instance, for compound I, divide -22 by 4 to obtain -5.5.
Continue for the rest of the compounds. For compound II, divide -22 by 3 to get -7.3. For compound III, divide -39 by 4 to get -9.75. For compound IV, divide -13 by 3 to get -4.3. If the powers are within one of each other, then the shortcut should not be used but rather the full math.
Remember that these numbers represent negative exponents. Depending on what the question is asking for, the correct order can be IV → I → II → III or III →→right arrow III → lI → I → IV
The question stem is asking for increasing pH, which means increasing basicity or OH⁻ concentration. Compound III has a molar solubility of about 10−9.75 while compound IV about 10−4.3
Since compound III has the lowest molar solubility, the correct order is the following: III → lI → I → IV
AgBr is a sparingly soluble salt and has a Ksp value of 5 x 10−13 By adding thiosulfate, we are able to dramatically increase the solubility of AgBr. Given the equation and equilibrium constant below, what is the formation constant (Kf) of the complex ion [Ag(S2O3)2]2-?,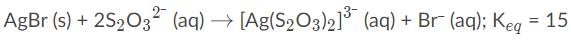
- a)6.7 x 1012
- b)3.0 x 1013
- c)7.5 x 10-12
- d)15
Correct answer is option 'B'. Can you explain this answer?
AgBr is a sparingly soluble salt and has a Ksp value of 5 x 10−13 By adding thiosulfate, we are able to dramatically increase the solubility of AgBr. Given the equation and equilibrium constant below, what is the formation constant (Kf) of the complex ion [Ag(S2O3)2]2-?,
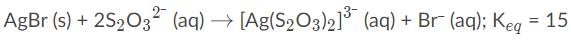
a)
6.7 x 1012
b)
3.0 x 1013
c)
7.5 x 10-12
d)
15

|
Orion Classes answered |
Let’s write out the equation for the solubility product constant:
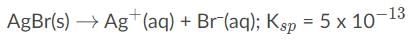
The formation reaction of a complex ion consists of the metal ion and the ligands on the reactant side and the complex ion on the product side:
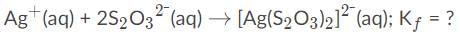
Combining the two equations together produces the original equation:
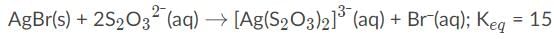
When adding or combining equations together, the equilibrium constants must be multiplied. So here is the correct answer:
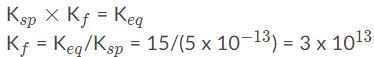
When the value of Kc is very small then- a)reaction is at start
- b)reactant conc. Is maximum
- c)reactant conc. Is minimum
- d)reaction is completed
Correct answer is option 'B'. Can you explain this answer?
When the value of Kc is very small then
a)
reaction is at start
b)
reactant conc. Is maximum
c)
reactant conc. Is minimum
d)
reaction is completed
|
|
Rutuja Desai answered |
When the value of Kc is very small, it indicates that the equilibrium position of the reaction lies towards the left side, meaning that the concentration of the reactants is relatively high compared to the concentration of the products.
Let's understand this in detail:
Equilibrium Constant (Kc):
The equilibrium constant, Kc, is a measure of the extent of a chemical reaction at equilibrium. It is defined as the ratio of the product concentrations to the reactant concentrations, each raised to their respective stoichiometric coefficients, at equilibrium, with each concentration term raised to the power equal to its stoichiometric coefficient in the balanced chemical equation.
Kc = [Products]^n / [Reactants]^m
Where [Products] and [Reactants] represent the concentrations of the products and reactants at equilibrium, respectively, and n and m are the stoichiometric coefficients of the products and reactants, respectively.
Interpretation of a Very Small Kc Value:
When the value of Kc is very small, it means that the numerator (concentration of products) is much smaller compared to the denominator (concentration of reactants).
This implies that the concentration of the reactants is significantly higher than the concentration of the products at equilibrium.
Explanation of Correct Answer (Option B):
The correct answer, "reactant conc. is maximum," indicates that when the value of Kc is very small, the concentration of the reactants is at its maximum.
This is because the equilibrium position favors the reactants, and the reaction is not proceeding significantly towards the product side. As a result, the concentration of the reactants remains high, while the concentration of the products is relatively low.
In other words, the reaction is not proceeding to a significant extent, and the reactants are dominant in the equilibrium mixture.
Therefore, when the value of Kc is very small, it signifies that the reaction is at the start, and the concentration of the reactants is maximum.
Let's understand this in detail:
Equilibrium Constant (Kc):
The equilibrium constant, Kc, is a measure of the extent of a chemical reaction at equilibrium. It is defined as the ratio of the product concentrations to the reactant concentrations, each raised to their respective stoichiometric coefficients, at equilibrium, with each concentration term raised to the power equal to its stoichiometric coefficient in the balanced chemical equation.
Kc = [Products]^n / [Reactants]^m
Where [Products] and [Reactants] represent the concentrations of the products and reactants at equilibrium, respectively, and n and m are the stoichiometric coefficients of the products and reactants, respectively.
Interpretation of a Very Small Kc Value:
When the value of Kc is very small, it means that the numerator (concentration of products) is much smaller compared to the denominator (concentration of reactants).
This implies that the concentration of the reactants is significantly higher than the concentration of the products at equilibrium.
Explanation of Correct Answer (Option B):
The correct answer, "reactant conc. is maximum," indicates that when the value of Kc is very small, the concentration of the reactants is at its maximum.
This is because the equilibrium position favors the reactants, and the reaction is not proceeding significantly towards the product side. As a result, the concentration of the reactants remains high, while the concentration of the products is relatively low.
In other words, the reaction is not proceeding to a significant extent, and the reactants are dominant in the equilibrium mixture.
Therefore, when the value of Kc is very small, it signifies that the reaction is at the start, and the concentration of the reactants is maximum.
A mixture of NH4Cl and NH4OH shows no change in pH upon addition small amount of HCl. This is because it is- a)A neutral solution
- b)A buffer
- c)A basic solution due to presence of NH4OH
- d)An acidic solution due to presence of NH4Cl
Correct answer is option 'B'. Can you explain this answer?
A mixture of NH4Cl and NH4OH shows no change in pH upon addition small amount of HCl. This is because it is
a)
A neutral solution
b)
A buffer
c)
A basic solution due to presence of NH4OH
d)
An acidic solution due to presence of NH4Cl
|
|
Rajat Patel answered |
A mixture of weak base and its salt with a strong acid serves as an basic buffer which resists changes in pH upon addition of small amount of acid or base.
Which of the following statements most accurately describes the data in the following chart depicting solubility of various compounds in water at varying temperatures?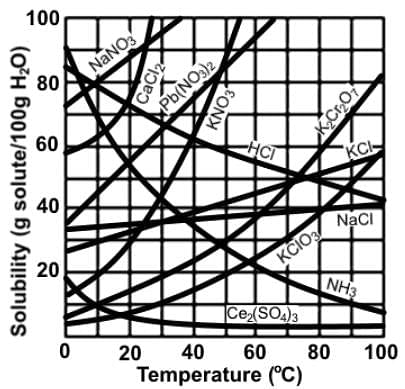
- a)The solubility of solids increases as temperature increases because the dissolution of solids is an exothermic process.
- b)The solubility of gases decreases as temperature increases because the dissolution of gas is an endothermic process.
- c)The solubility of HCl decreases due to the extensive hydrogen bonding that occurs between HCl molecules.
- d)The solubility of cesium sulfate decreases since the enthalpy of hydration releases more heat than is needed for the lattice dissociation energy.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements most accurately describes the data in the following chart depicting solubility of various compounds in water at varying temperatures?

a)
The solubility of solids increases as temperature increases because the dissolution of solids is an exothermic process.
b)
The solubility of gases decreases as temperature increases because the dissolution of gas is an endothermic process.
c)
The solubility of HCl decreases due to the extensive hydrogen bonding that occurs between HCl molecules.
d)
The solubility of cesium sulfate decreases since the enthalpy of hydration releases more heat than is needed for the lattice dissociation energy.

|
Orion Classes answered |
If we look at the chart, all the gases (HCl and NH3) decreases in solubility as the temperature increases. When gases dissolve in a liquid, it is an exothermic process due to the latent heat released when the gas is brought from the gaseous to liquid or aqueous phase.
As for HCl, there is no hydrogen bonding since only F, O, and N can H-bond. Additionally, gas molecules do not H-bond since the particles are far apart.
Most of the solids increases its solubility as the temperature increase because the dissolution of solids is an endothermic process. Heat would be on the reactant side, and by adding more reactant, the equilibrium shifts to the right.
When considering the dissolution of solids, there are 2 possibilities: endothermic and exothermic. When dissolution is exothermic, more will not dissolve when the temperature increases.
For cesium sulfate, the heat released when new bonds are formed between the ions and water molecules or the enthalpy of hydration is very exothermic. It exceeds the energy required to break the lattice, which is equal to the lattice energy.
The correct answer is that the solubility of cesium sulfate decreases since the enthalpy of hydration releases more heat than is needed for the lattice dissociation energy.
Which of the following is an example of basic buffer?- a)A mixture of HCl and NaOH
- b)A mixture of NH4Cl and NH4OH
- c)A mixture of CH3COOH and OH3COONa
- d)1M solution of NaOH
Correct answer is option 'B'. Can you explain this answer?
Which of the following is an example of basic buffer?
a)
A mixture of HCl and NaOH
b)
A mixture of NH4Cl and NH4OH
c)
A mixture of CH3COOH and OH3COONa
d)
1M solution of NaOH
|
|
Nandini Iyer answered |
Acidic buffer solution contains equimolar quantities of a weak acid and its salt with strong base. For example: an acetic acid, CH3COOH and sodium acetate I.e. CH3COONa. Basic buffer solution contains equimolar quantities of a weak base and its salt with strong acid.
Which of the following compounds will not create a precipitate upon addition into a saturated solution of potassium nitrate?- a)KOH
- b)Ag2SO4
- c)Hg2(NO3)2
- d)NH4NO3
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds will not create a precipitate upon addition into a saturated solution of potassium nitrate?
a)
KOH
b)
Ag2SO4
c)
Hg2(NO3)2
d)
NH4NO3
|
|
Ayesha Joshi answered |
We are starting with a saturated solution of potassium nitrate rather than pure water. A saturated solution is a solution in which the maximum amount of solute has been dissolved.
That means more of neither the potassium cation or nitrate anion can be dissolved into the solution. Any compounds with either the potassium or nitrate ion will cause precipitate to form.
KOH, Hg2(NO3)2 and NH4NO3 will cause a precipitate to form, and only Ag2SO4 will not cause precipitate to form.
Based on general solubility rules, which of the following compounds is considered soluble?- a)Cs2CrO4
- b)PbCl2
- c)Al(OH)3
- d)CaSO4
Correct answer is option 'A'. Can you explain this answer?
Based on general solubility rules, which of the following compounds is considered soluble?
a)
Cs2CrO4
b)
PbCl2
c)
Al(OH)3
d)
CaSO4
|
|
Evelyn Clark answered |
Understanding Solubility Rules
Solubility rules help determine the solubility of various compounds in water. Among the given options, we will explore why Cs2CrO4 is considered soluble.
General Solubility Guidelines
- Alkali Metal Compounds: Compounds containing alkali metals (Group 1 elements) are generally soluble.
- Nitrates and Acetates: Compounds containing nitrates (NO3-) and acetates (C2H3O2-) are soluble.
- Sulfates: Most sulfate salts are soluble, but there are exceptions.
- Hydroxides and Carbonates: Generally insoluble, with some exceptions for alkali metals and certain alkaline earth metals.
Analysis of Each Compound
- Cs2CrO4 (Cesium Chromate):
- Contains cesium (an alkali metal).
- According to solubility rules, all salts of alkali metals are soluble.
- Therefore, Cs2CrO4 is soluble.
- PbCl2 (Lead(II) Chloride):
- While chlorides are generally soluble, lead(II) chloride is an exception; it is only slightly soluble.
- Al(OH)3 (Aluminum Hydroxide):
- Hydroxides are typically insoluble, and aluminum hydroxide is one of them.
- CaSO4 (Calcium Sulfate):
- Calcium sulfate is generally considered only slightly soluble in water.
Conclusion
Based on the solubility rules, Cs2CrO4 is the only soluble compound among the options provided, confirming it as the correct answer.
Solubility rules help determine the solubility of various compounds in water. Among the given options, we will explore why Cs2CrO4 is considered soluble.
General Solubility Guidelines
- Alkali Metal Compounds: Compounds containing alkali metals (Group 1 elements) are generally soluble.
- Nitrates and Acetates: Compounds containing nitrates (NO3-) and acetates (C2H3O2-) are soluble.
- Sulfates: Most sulfate salts are soluble, but there are exceptions.
- Hydroxides and Carbonates: Generally insoluble, with some exceptions for alkali metals and certain alkaline earth metals.
Analysis of Each Compound
- Cs2CrO4 (Cesium Chromate):
- Contains cesium (an alkali metal).
- According to solubility rules, all salts of alkali metals are soluble.
- Therefore, Cs2CrO4 is soluble.
- PbCl2 (Lead(II) Chloride):
- While chlorides are generally soluble, lead(II) chloride is an exception; it is only slightly soluble.
- Al(OH)3 (Aluminum Hydroxide):
- Hydroxides are typically insoluble, and aluminum hydroxide is one of them.
- CaSO4 (Calcium Sulfate):
- Calcium sulfate is generally considered only slightly soluble in water.
Conclusion
Based on the solubility rules, Cs2CrO4 is the only soluble compound among the options provided, confirming it as the correct answer.
Which of the following statements most accurately describes the solubility product constant?- a)The most direct measure of how much a solid dissolves is the solubility product constant.
- b)When the solubility product is greater than the ion product, the solution is considered supersaturated.
- c)The molar solubility of a solid is always less than the solubility product constant.
- d)Each concentration in the Ksp expression is raised to the power of the charge of the opposing ion.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements most accurately describes the solubility product constant?
a)
The most direct measure of how much a solid dissolves is the solubility product constant.
b)
When the solubility product is greater than the ion product, the solution is considered supersaturated.
c)
The molar solubility of a solid is always less than the solubility product constant.
d)
Each concentration in the Ksp expression is raised to the power of the charge of the opposing ion.
|
|
Ayesha Joshi answered |
The direct measure of how much solid dissolves is the molar solubility which is expressed in moles solute per liter of solution. The solubility product constant represents the product of the concentrations of the ions present in solution.
The ion product is the product of the concentrations of the ions at any moment in time. When the ion product is greater than the solubility product constant, the solution is considered unsaturated
The molar solubility is always greater than the solubility product constant. Since we are dealing only with sparingly soluble solutes, the constant is expressed with negative exponents. Multiplying two numbers with negative exponents will always yield a smaller number.
Each concentration in the Ksp expression is raised to the stoichiometric coefficient, which happens to the same as the power of the charge of the opposing ion.
A mixture of CH3COOH and CH3COONa behaves as- a)Basic buffer
- b)Ionic buffer
- c)Acidic buffer
- d)Neutral buffer
Correct answer is option 'C'. Can you explain this answer?
A mixture of CH3COOH and CH3COONa behaves as
a)
Basic buffer
b)
Ionic buffer
c)
Acidic buffer
d)
Neutral buffer
|
|
Pooja Mehta answered |
CH3COONH4 can act as a buffer solution as:-
When a strong acid is added to the solution, all the H+ ions take up CH3COO- and form acetic acid, CH3COOH which is a weakly dissociating acid.
When a strong base is added to the solution, all the OH- ions take NH4+ and form ammonium hydroxide, NH4OH which is a weakly dissociating base.
And we know that the salt of a weak acid and a weak base acts as buffer
Chapter doubts & questions for Solubility (GC) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Solubility (GC) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









