All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Enzymes (BC, BIO) for MCAT Exam
Which is true about enzymes?a)All enzymes are ... moreproteins.b)All proteins are enzymes.c)All enzymes are not proteins.d)All enzymes are vitamins.Correct answer is option 'C'. Can you explain this answer?
|
|
Gaurav Kumar answered |
Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. The latter are called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures.
Which one is true for ATP?- a)ATP is organic ions of enzyme.
- b)ATP is a coenzyme.
- c)ATP is an enzyme
- d)ATP is a prosthetic part of an enzyme.
Correct answer is option 'B'. Can you explain this answer?
Which one is true for ATP?
a)
ATP is organic ions of enzyme.
b)
ATP is a coenzyme.
c)
ATP is an enzyme
d)
ATP is a prosthetic part of an enzyme.
|
|
Hitakshi HTG answered |
Adenosine triphosphate, also known as molecular unit of currency, is a coenzyme of vast importance in the transfer of chemical energy derived from biochemical oxidations and it also transports energy within cells for metabolism. Thus, option 'B' is the right answer.
The enzyme which cuts DNA is- a)DNA polymerase
- b)DNA ligase
- c)Restriction endonucleases
- d)DNA lyase
Correct answer is option 'C'. Can you explain this answer?
The enzyme which cuts DNA is
a)
DNA polymerase
b)
DNA ligase
c)
Restriction endonucleases
d)
DNA lyase
|
|
Baby Ghosh answered |
Restriction Endonucleases are enzyme which scan DNA molecules for a particular nucleotide sequence. These are called Recognition Sequences.Once the Endonuclease finds this sequence it halts ans cuts the strand.Thus,the correct option is "C".
The enzyme found functional in lysosome is- a)Acid phosphataseLyases
- b)Lyases
- c)Oxidoreductase
- d)Basic phosphatase
Correct answer is option 'B'. Can you explain this answer?
The enzyme found functional in lysosome is
a)
Acid phosphatase
Lyases
b)
Lyases
c)
Oxidoreductase
d)
Basic phosphatase
|
|
Vijay Bansal answered |
A lysosome is a membrane-bound organelle found in nearly all animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules.
Which enzyme is concerned with the transfer of electrons?
- a)Dehydrogenase
- b)Transaminase
- c)Hydrolase
- d) Desmolase
Correct answer is option 'A'. Can you explain this answer?
Which enzyme is concerned with the transfer of electrons?
a)
Dehydrogenase
b)
Transaminase
c)
Hydrolase
d)
Desmolase
|
|
Leelu Bhai answered |
Transaminase are the enzymes concerned with transfer of atoms or group of atoms or electrons.. so, option B is correct not A
Enzyme amylase belongs to- a)Hydrolases
- b)Transferases
- c)Isomerases
- d)Oxidoreductases
Correct answer is option 'A'. Can you explain this answer?
Enzyme amylase belongs to
a)
Hydrolases
b)
Transferases
c)
Isomerases
d)
Oxidoreductases
|
|
Ragini Shukla answered |
Hydrolase are the enzyme which breaks down the large molecules into smaller ones with the help of hydrogen and hydroxyl groups of water molecule this phenomena is known as hydrolysis. Amylase is the enzyme produced by the salivary gland and helps in breaking down the starch into glucose. Amylase catalysis the hydrolysis of starch into sugar (glucose).
Amylase is also found in germinating seeds.
Amylase is also found in germinating seeds.
Enzymes with slightly different molecular structure but performing identical activity are- a)Isoenzymes
- b)Apoenzymes
- c)Holoenzyme
- d)Coenzymes
Correct answer is option 'A'. Can you explain this answer?
Enzymes with slightly different molecular structure but performing identical activity are
a)
Isoenzymes
b)
Apoenzymes
c)
Holoenzyme
d)
Coenzymes
|
|
Mira Tiwari answered |
Enzymes with slightly different molecular structure but performing identical activity are called isoenzymes.
Explanation:
Enzymes are biological catalysts that facilitate and speed up biochemical reactions in living organisms. They are usually proteins with a specific three-dimensional structure that allows them to bind to specific substrates and convert them into products. However, enzymes can also have slightly different molecular structures while performing the same catalytic activity. These enzymes are called isoenzymes.
Definition of Isoenzymes:
Isoenzymes, also known as isozymes, are enzymes that catalyze the same chemical reaction but have slightly different amino acid sequences or molecular structures. They are encoded by different genes but perform identical functions in the same organism or different tissues. Isoenzymes are often found in multiple forms and can be distinguished from each other through various methods such as electrophoresis or immunoassay.
Reasons for Different Molecular Structures:
1. Genetic Differences: Isoenzymes are encoded by different genes, which can result in variations in the amino acid sequence and overall structure of the enzyme.
2. Post-translational Modifications: Isoenzymes may undergo different post-translational modifications, such as phosphorylation or glycosylation, which can alter their structure and function.
3. Tissue-Specific Expression: Different tissues within an organism may require slightly different versions of an enzyme to perform the same activity optimally. These tissue-specific isoenzymes may have distinct molecular structures.
Importance of Isoenzymes:
1. Diagnostic Tool: Isoenzymes can be used as diagnostic markers for certain diseases or conditions. For example, the measurement of different forms of creatine kinase isoenzymes can help diagnose heart attacks.
2. Tissue-Specific Function: Isoenzymes may have different kinetic properties or substrate specificities, allowing them to perform specialized functions in specific tissues.
3. Evolutionary Adaptation: Isoenzymes can evolve to perform similar functions under different conditions or in different organisms, allowing for adaptation and survival in diverse environments.
In conclusion, isoenzymes refer to enzymes with slightly different molecular structures but identical catalytic activities. They play important roles in various biological processes and have diagnostic and evolutionary significance.
Explanation:
Enzymes are biological catalysts that facilitate and speed up biochemical reactions in living organisms. They are usually proteins with a specific three-dimensional structure that allows them to bind to specific substrates and convert them into products. However, enzymes can also have slightly different molecular structures while performing the same catalytic activity. These enzymes are called isoenzymes.
Definition of Isoenzymes:
Isoenzymes, also known as isozymes, are enzymes that catalyze the same chemical reaction but have slightly different amino acid sequences or molecular structures. They are encoded by different genes but perform identical functions in the same organism or different tissues. Isoenzymes are often found in multiple forms and can be distinguished from each other through various methods such as electrophoresis or immunoassay.
Reasons for Different Molecular Structures:
1. Genetic Differences: Isoenzymes are encoded by different genes, which can result in variations in the amino acid sequence and overall structure of the enzyme.
2. Post-translational Modifications: Isoenzymes may undergo different post-translational modifications, such as phosphorylation or glycosylation, which can alter their structure and function.
3. Tissue-Specific Expression: Different tissues within an organism may require slightly different versions of an enzyme to perform the same activity optimally. These tissue-specific isoenzymes may have distinct molecular structures.
Importance of Isoenzymes:
1. Diagnostic Tool: Isoenzymes can be used as diagnostic markers for certain diseases or conditions. For example, the measurement of different forms of creatine kinase isoenzymes can help diagnose heart attacks.
2. Tissue-Specific Function: Isoenzymes may have different kinetic properties or substrate specificities, allowing them to perform specialized functions in specific tissues.
3. Evolutionary Adaptation: Isoenzymes can evolve to perform similar functions under different conditions or in different organisms, allowing for adaptation and survival in diverse environments.
In conclusion, isoenzymes refer to enzymes with slightly different molecular structures but identical catalytic activities. They play important roles in various biological processes and have diagnostic and evolutionary significance.
Which of these enzymes are not proteinaceous?- a)Ribozymes
- b)Endonucleases
- c)Ligases
- d)Kinases
Correct answer is option 'A'. Can you explain this answer?
Which of these enzymes are not proteinaceous?
a)
Ribozymes
b)
Endonucleases
c)
Ligases
d)
Kinases
|
|
Gaurav Basu answered |
Explanation:
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They are typically protein molecules, but there are exceptions.
Ribozymes
Ribozymes are RNA molecules that exhibit catalytic activity. Unlike most enzymes, which are proteinaceous, ribozymes are composed of RNA. They were first discovered in the 1980s and have since been found to play important roles in various biological processes. Ribozymes can catalyze a wide range of reactions, including cleavage, ligation, and isomerization reactions.
Endonucleases
Endonucleases are enzymes that cleave the phosphodiester bonds within a nucleic acid chain. They are involved in processes such as DNA replication, repair, recombination, and transcription. Endonucleases can be either proteinaceous or ribozymes.
Ligases
Ligases are enzymes that catalyze the joining of two molecules by forming a new chemical bond, usually in the presence of ATP. They are involved in DNA repair, DNA replication, and the synthesis of RNA and proteins. Ligases are proteinaceous enzymes.
Kinases
Kinases are enzymes that catalyze the transfer of a phosphate group from ATP to a substrate molecule, a process known as phosphorylation. This phosphorylation can regulate the activity of the substrate molecule. Kinases are proteinaceous enzymes.
Conclusion
Among the enzymes listed, ribozymes are not proteinaceous. Ribozymes are RNA molecules that exhibit catalytic activity and can perform a wide range of enzymatic reactions. This discovery challenged the long-held belief that all enzymes are proteinaceous. Ribozymes have since been found to play important roles in various biological processes, contributing to our understanding of the diversity and complexity of enzymatic reactions in living organisms.
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They are typically protein molecules, but there are exceptions.
Ribozymes
Ribozymes are RNA molecules that exhibit catalytic activity. Unlike most enzymes, which are proteinaceous, ribozymes are composed of RNA. They were first discovered in the 1980s and have since been found to play important roles in various biological processes. Ribozymes can catalyze a wide range of reactions, including cleavage, ligation, and isomerization reactions.
Endonucleases
Endonucleases are enzymes that cleave the phosphodiester bonds within a nucleic acid chain. They are involved in processes such as DNA replication, repair, recombination, and transcription. Endonucleases can be either proteinaceous or ribozymes.
Ligases
Ligases are enzymes that catalyze the joining of two molecules by forming a new chemical bond, usually in the presence of ATP. They are involved in DNA repair, DNA replication, and the synthesis of RNA and proteins. Ligases are proteinaceous enzymes.
Kinases
Kinases are enzymes that catalyze the transfer of a phosphate group from ATP to a substrate molecule, a process known as phosphorylation. This phosphorylation can regulate the activity of the substrate molecule. Kinases are proteinaceous enzymes.
Conclusion
Among the enzymes listed, ribozymes are not proteinaceous. Ribozymes are RNA molecules that exhibit catalytic activity and can perform a wide range of enzymatic reactions. This discovery challenged the long-held belief that all enzymes are proteinaceous. Ribozymes have since been found to play important roles in various biological processes, contributing to our understanding of the diversity and complexity of enzymatic reactions in living organisms.
An organic substance bound to an enzyme and essential for its activity is called- a)Isoenzyme
- b)Coenzyme
- c)Holoenzyme
- d)Apoenzyme
Correct answer is option 'B'. Can you explain this answer?
An organic substance bound to an enzyme and essential for its activity is called
a)
Isoenzyme
b)
Coenzyme
c)
Holoenzyme
d)
Apoenzyme
|
|
Rohan Singh answered |
Coenzyme is an organic nonprotein molecule that associates with an enzyme molecule in catalsying biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups. Holoenzyme is a complex comprising of enzyme molecule and its cofactor. The enzyme is catalytically active in this state. Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function. Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzyme-substrate reaction.
At which part of the enzyme does the substrate fit in?- a)Active site
- b)Right end
- c)Left end
- d)Binding site
Correct answer is option 'A'. Can you explain this answer?
At which part of the enzyme does the substrate fit in?
a)
Active site
b)
Right end
c)
Left end
d)
Binding site
|
|
Megha Basu answered |
Active site:
The substrate fits into the active site of an enzyme. The active site is a specific region on the enzyme where the substrate binds and undergoes a chemical reaction. This site has a unique shape and chemical properties that complement the structure of the substrate molecule.
Key points:
- The active site is like a lock and key, where the substrate (key) fits into the active site (lock) with precision.
- The active site provides a microenvironment that is conducive to the chemical reaction between the enzyme and substrate.
- The binding of the substrate to the active site induces a conformational change in the enzyme, which helps facilitate the reaction.
- The active site may contain specific amino acid residues that directly participate in the catalytic process.
In conclusion, the substrate fits into the active site of an enzyme, where it undergoes a chemical reaction facilitated by the enzyme's specific structure and properties.
The substrate fits into the active site of an enzyme. The active site is a specific region on the enzyme where the substrate binds and undergoes a chemical reaction. This site has a unique shape and chemical properties that complement the structure of the substrate molecule.
Key points:
- The active site is like a lock and key, where the substrate (key) fits into the active site (lock) with precision.
- The active site provides a microenvironment that is conducive to the chemical reaction between the enzyme and substrate.
- The binding of the substrate to the active site induces a conformational change in the enzyme, which helps facilitate the reaction.
- The active site may contain specific amino acid residues that directly participate in the catalytic process.
In conclusion, the substrate fits into the active site of an enzyme, where it undergoes a chemical reaction facilitated by the enzyme's specific structure and properties.
Which of the following is a Lyase? (JIPMER 2014)- a)Aldolase B
- b)Acetyl Co ASynthetase
- c)Fatty Acyl CoA Dehydrogenase
- d)Acetyl CoA Carboxylase
Correct answer is option 'A'. Can you explain this answer?
a)
Aldolase B
b)
Acetyl Co ASynthetase
c)
Fatty Acyl CoA Dehydrogenase
d)
Acetyl CoA Carboxylase

|
Ciel Knowledge answered |
- Examples of lyases include: HMG CoA Lyase
- Argininosuccinate Lyase
- ATP Citrate Lyase
- Aldolase
- Fumarase.
Noncompetitive enzyme inhibition leads to:- a)Vmax ↑
- b)Vmax ↓
- c)Vmax unchanged
- d)Km ↑
- e)Km ↓
Correct answer is option 'B'. Can you explain this answer?
Noncompetitive enzyme inhibition leads to:
a)
Vmax ↑
b)
Vmax ↓
c)
Vmax unchanged
d)
Km ↑
e)
Km ↓

|
Ciel Knowledge answered |
- Characteristics of noncompetitive inhibition:
- The inhibitor lacks any structural similarity to the substrate.
- It is irreversible.
- An excess of substrate does not eliminate the inhibition.
- The value of Km remains unchanged.
- The maximum velocity (Vmax) decreases.
All are true about oxygenases, except: (AIIMS Nov 2011)- a)Can incorporate 2 atoms of O, in a substance
- b)Can incorporate 1 atom of O, in a substance
- c)Important in hydroxylation of steroids
- d)Catalyse carboxylation of drugs
Correct answer is option 'D'. Can you explain this answer?
a)
Can incorporate 2 atoms of O, in a substance
b)
Can incorporate 1 atom of O, in a substance
c)
Important in hydroxylation of steroids
d)
Catalyse carboxylation of drugs

|
Ciel Knowledge answered |
Oxygenases can be classified as either monooxygenases or dioxygenases.
- Monooxygenases incorporate one atom of an oxygen molecule into the substrate.
- Dioxygenases incorporate both atoms of an oxygen molecule into the substrate.
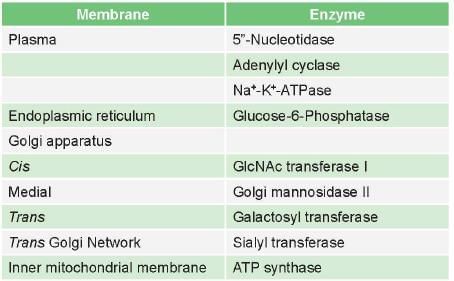
Markers of plasma membrane is/are: (PGI June 2009)- a)Galactosyltransferase
- b)5'nucleotidase
- c)Adenyl cyclase
- d)ATP synthetase
- e)Tyrosine
Correct answer is option 'B,C'. Can you explain this answer?
Markers of plasma membrane is/are: (PGI June 2009)
a)
Galactosyltransferase
b)
5'nucleotidase
c)
Adenyl cyclase
d)
ATP synthetase
e)
Tyrosine

|
Ciel Knowledge answered |
Enzymes as markers of organelle and membranes
Enzymatic markers of different membranes
Enzymatic markers of different membranes
Km changes and Vmax remains the same. What is the type of Enzyme inhibition?- a)Competitive Inhibition
- b)Noncompetitive Inhibition
- c)Uncompetitive inhibition
- d)Suicide Inhibition
Correct answer is option 'A'. Can you explain this answer?
Km changes and Vmax remains the same. What is the type of Enzyme inhibition?
a)
Competitive Inhibition
b)
Noncompetitive Inhibition
c)
Uncompetitive inhibition
d)
Suicide Inhibition

|
Ciel Knowledge answered |
- Competitive inhibition - Km rises while Vmax stays unchanged.
- Noncompetitive inhibition - Km remains constant, but Vmax decreases.
- Uncompetitive inhibition - Both Km and Vmax decline.
True about isoenzymes is: (AIIMS Nov 2011)- a)Catalyse the same reaction
- b)Same quaternary structure
- c)Same distribution in different organs
- d)Same enzyme classification with same number and name
Correct answer is option 'A'. Can you explain this answer?
a)
Catalyse the same reaction
b)
Same quaternary structure
c)
Same distribution in different organs
d)
Same enzyme classification with same number and name

|
Ciel Knowledge answered |
Isoenzymes facilitate identical reactions. For instance, LDH1-5 all convert pyruvate to lactate.
- They exhibit distinct quaternary structures.
- The subunits in LDH1 differ from those in LDH2.
- The tissue distribution of each isoform varies.
- The enzyme names and numbers may also differ.
Chymotrypsin cleaves carbonyl terminal of: (PGI May 2011)- a)Phenylalanine
- b)Arginine
- c)Lysine
- d)Tryptophan
- e)Tyrosine
Correct answer is option 'A,D,E'. Can you explain this answer?
a)
Phenylalanine
b)
Arginine
c)
Lysine
d)
Tryptophan
e)
Tyrosine

|
Ciel Knowledge answered |
Serine proteases are a type of proteolytic enzyme characterised by the presence of serine at their active site. The amino acid triad located in the active site of serine proteases consists of:
- Ser
- His
- Asp
Examples of serine proteases include:
- Chymotrypsin
- Trypsin
- Elastase
- Thrombin
- Plasmin
- Complement Factors X and XL
Serine proteases exhibit variations in substrate specificity:
- Trypsin cleaves basic amino acids, such as Arg and Lys.
- Chymotrypsin cleaves hydrophobic, bulky amino acids like Trp, Tyr, and Phe.
- Elastase cleaves small, neutral amino acids such as Alanine and Glycine.
All of the covalent modification regulate enzyme kinetics except:- a)Phosphorylation
- b)Acetylation
- c)ADP Ribosylation
- d)Glycosylation
Correct answer is option 'D'. Can you explain this answer?
a)
Phosphorylation
b)
Acetylation
c)
ADP Ribosylation
d)
Glycosylation

|
Ciel Knowledge answered |
Reversible covalent modifications include
- Phosphorylation
- Dephosphorylation
- ADP Ribosylation
- Methylation
- Acetylation
- Zymogen activation
- Partial proteolysis
An organic substance bound to an enzyme and essential for its activity is called- a)Isoenzyme
- b)Coenzyme
- c)Holoenzyme
- d)Apoenzyme
Correct answer is option 'B'. Can you explain this answer?
An organic substance bound to an enzyme and essential for its activity is called
a)
Isoenzyme
b)
Coenzyme
c)
Holoenzyme
d)
Apoenzyme

|
Arathy Arab answered |
B. coenzyme
Coenzyme is an organic nonprotein molecule that associates with an enzymes molecule in catalysing biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups.
Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function.
Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzymes-substrate reaction.
Coenzyme is an organic nonprotein molecule that associates with an enzymes molecule in catalysing biochemical reactions. It usually participates in the substrate-enzyme interaction by donating or accepting certain chemical groups.
Apoenzyme is an inactive enzyme that must associate with a specific cofactor molecule in order to function.
Isoenzyme or isozyme is one of the several forms of an enzyme that catalyse the same reaction but differ from each other in such properties as substrate affinity and maximum rates of enzymes-substrate reaction.
How are enzymes different from catalysts?- a)Enzymes are active at high temperatures
- b)Catalysts are active at subzero temperatures
- c)Catalysts are efficient at high temperatures and high pressures
- d)Enzymes are denatured at room temperature
Correct answer is option 'C'. Can you explain this answer?
How are enzymes different from catalysts?
a)
Enzymes are active at high temperatures
b)
Catalysts are active at subzero temperatures
c)
Catalysts are efficient at high temperatures and high pressures
d)
Enzymes are denatured at room temperature

|
Top Rankers answered |
- Enzymes are organic catalysts or biocatalysts.
- However, inorganic catalysts work at high temperatures and high pressures efficiently.
- Enzymes, being proteinaceous in nature, get denatured under such conditions.
Which is true about enzyme kinetics for competitive inhibition: (JIPMER 2014)- a)Low km high affinity
- b)High km high affinity
- c)High Km low affinity
- d)Low Km low affinity
Correct answer is option 'C'. Can you explain this answer?
Which is true about enzyme kinetics for competitive inhibition: (JIPMER 2014)
a)
Low km high affinity
b)
High km high affinity
c)
High Km low affinity
d)
Low Km low affinity

|
Ciel Knowledge answered |
Km (Michaelis Constant) represents the substrate concentration at which the reaction rate is half of Vmax for a given enzyme-substrate combination. It serves as an identifier for the enzyme:
- A higher Km indicates a lower affinity of the enzyme for the substrate.
- A lower Km signifies a higher affinity of the enzyme for the substrate.
Characteristics of competitive inhibition include:
- Km increases, resulting in reduced affinity.
- Vmax remains unchanged.
Characteristics of noncompetitive inhibition include:
- Km remains constant.
- Vmax decreases.
Coenzyme in decarboxylation reaction:- a)Niacin
- b)Biotin
- c)Pyridoxine
- d)Riboflavin
Correct answer is option 'C'. Can you explain this answer?
a)
Niacin
b)
Biotin
c)
Pyridoxine
d)
Riboflavin

|
Ciel Knowledge answered |
- Biotin serves as a coenzyme for carboxylation reactions.
- Pyridoxine acts as a coenzyme for decarboxylation.
- Thiamine functions as a coenzyme for the decarboxylation processes involving Alpha-ketoglutarate dehydrogenase and branched-chain ketoacid dehydrogenase.
Chymotrypsinogen is a:- a)Zymogen
- b)Carboxypeptidase
- c)Transaminase
- d)Exopeptidase
Correct answer is option 'A'. Can you explain this answer?
a)
Zymogen
b)
Carboxypeptidase
c)
Transaminase
d)
Exopeptidase

|
Ciel Knowledge answered |
Zymogen activation represents a case of irreversible covalent alteration.
The following affect enzyme activity except:- a)Methylation
- b)Acetylation
- c)Induction
- d)Phosphorylation
Correct answer is option 'C'. Can you explain this answer?
a)
Methylation
b)
Acetylation
c)
Induction
d)
Phosphorylation

|
Ciel Knowledge answered |
Induction refers to the process of regulating the amount of enzymes. The regulation of enzymes can be categorised into:
- Regulation of Enzyme Quality (Intrinsic Catalytic Efficiency)
- Allosteric Regulation
- Covalent Modification
- Phosphorylation/dephosphorylation (the most prevalent covalent modification)
- Methylation
- Adenylation
- ADP ribosylation
- Acetylation
- Control of Enzyme Synthesis through Induction and Repression
- Control of Enzyme Degradation
Feedback inhibition of enzymes is affected by which of the following:- a)Enzyme
- b)End-products
- c)Substrate
- d)Intermediate and products
Correct answer is option 'B'. Can you explain this answer?
Feedback inhibition of enzymes is affected by which of the following:
a)
Enzyme
b)
End-products
c)
Substrate
d)
Intermediate and products
|
|
Jananignanamurugan Janan answered |
Yes option B is correct
when the end products levels are more,products will bind to enzymes and reduces its activity.After the body utilises products and the level decreases and becomes zero means then enzymes become free from inhibitors(products) and then again the enzymes catalyse the reaction and form products
when the end products levels are more,products will bind to enzymes and reduces its activity.After the body utilises products and the level decreases and becomes zero means then enzymes become free from inhibitors(products) and then again the enzymes catalyse the reaction and form products
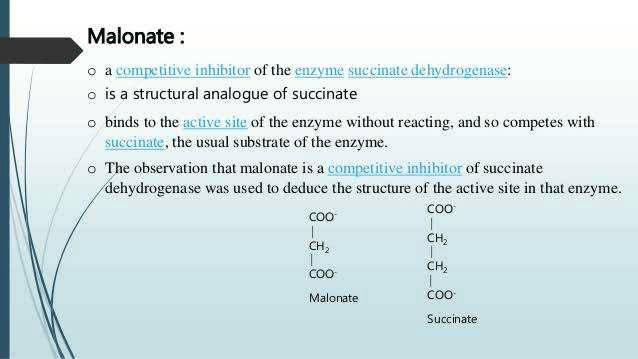
The type of enzyme inhibition in which Succinate dehydrogenase reaction is inhibited by malonate is an example of: (AIIMS May 2006)- a)Noncompetitive
- b)Uncompetitive
- c)Competitive
- d)Allosteric
Correct answer is option 'C'. Can you explain this answer?
The type of enzyme inhibition in which Succinate dehydrogenase reaction is inhibited by malonate is an example of: (AIIMS May 2006)
a)
Noncompetitive
b)
Uncompetitive
c)
Competitive
d)
Allosteric

|
Ciel Knowledge answered |
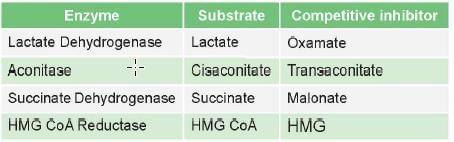
Examples of competitive inhibition
Competitive inhibitors of enzymes are mostly drugs
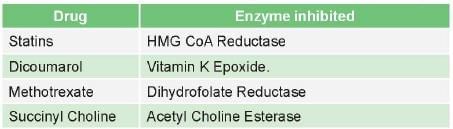
Some competitive inhibitor which are not drugs are
Features of competitive inhibition is/are: (PGI May 2014)- a)Vmax increases
- b)Km increases
- c)Vmax decreases
- d)Km decreases
- e)Vmax constant
Correct answer is option 'B,E'. Can you explain this answer?
Features of competitive inhibition is/are: (PGI May 2014)
a)
Vmax increases
b)
Km increases
c)
Vmax decreases
d)
Km decreases
e)
Vmax constant

|
Ciel Knowledge answered |
Characteristics of competitive inhibition include:
- V remains a constant max
- Km increases
- It is reversible.
All of the following enzymes are involved in oxidation-reduction, except: (AI 2009)- a)Dehydrogenases
- b)Hydrolases
- c)Oxygenases
- d)Peroxidases
Correct answer is option 'B'. Can you explain this answer?
a)
Dehydrogenases
b)
Hydrolases
c)
Oxygenases
d)
Peroxidases

|
Ciel Knowledge answered |
Oxidoreductases can include:
- Dehydrogenase
- Oxygenase
- Monooxygenase
- Dioxygenase
- Oxidase
- Catalase
- Peroxidase
- Monoamine Oxidase
- Cytochrome Oxidase
- Xanthine Oxidase
- Phenylalanine Hydroxylases
- 7 alpha Hydroxylases
- Cytochrome p450
- A + O2 → AO2
- Homogentisate oxidase
- Tryptophan Pyrrolase (Dioxygenase)
- Nitric Oxide Synthase
Suicidal Enzyme is: (AIIMS May 2013)- a)Lipoxygenase
- b)Cyclooxygenase
- c)Thromboxane Synthase
- d)5' Nucleotidase
Correct answer is option 'B'. Can you explain this answer?
a)
Lipoxygenase
b)
Cyclooxygenase
c)
Thromboxane Synthase
d)
5' Nucleotidase

|
Ciel Knowledge answered |
Cyclooxygenase is referred to as a 'suicide enzyme.' The deactivation of prostaglandin activity is partially due to an exceptional characteristic of cyclooxygenase, which is its ability for self-catalysed destruction; in other words, it acts as a 'suicide enzyme.' Quick overview of cyclooxygenase:
- Cyclooxygenase (COX), also known as prostaglandin H synthase
- Plays a role in the synthesis of prostanoids (prostaglandins, thromboxane, and prostacyclin)
- Exhibits two activities: cyclooxygenase and peroxidase
- Exists as two isoenzymes: COX-1 and COX-2
- NSAIDs:
- Aspirin: Inhibits both COX-1 and COX-2
- Indomethacin and ibuprofen: Inhibit cyclooxygenases by competing with arachidonate
- Coxibs: Selectively inhibit COX-2; however, some have been withdrawn or suspended from the market due to adverse side effects and safety concerns
- Completely inhibit the transcription of COX-2, but not COX-1
Nonfunctional enzymes are all except: (AIIMS Nov 2008)- a)Alkaline phosphatase
- b)Acid phosphatese
- c)Lipoprotein lipase
- d)Gamma glutamyltranspeptidase
Correct answer is option 'C'. Can you explain this answer?
a)
Alkaline phosphatase
b)
Acid phosphatese
c)
Lipoprotein lipase
d)
Gamma glutamyltranspeptidase

|
Ciel Knowledge answered |
Functional enzymes refer to enzymes that serve specific roles within the plasma. Examples include:
- Coagulation Factors
- Lipoprotein Lipase
Non-functional enzymes do not have a specific role in serum. They:
- Are released from tissues due to normal wear and tear
- Exhibit very low levels in serum
- Increase in serum levels during tissue injury, aiding in the diagnosis of the injury site
Examples of non-functional enzymes include LDH, Creatine Kinase, and Alkaline Phosphatase.
Chapter doubts & questions for Enzymes (BC, BIO) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Enzymes (BC, BIO) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup