All Exams >
Mechanical Engineering >
SSC JE Mechanical Mock Test Series 2026 >
All Questions
All questions of Thermodynamics for Mechanical Engineering Exam
The standard fixed point of thermometry is- a)Ice point
- b)Sulphur point
- c)Triple point of water
- d)Normal boiling point of water
Correct answer is option 'C'. Can you explain this answer?
The standard fixed point of thermometry is
a)
Ice point
b)
Sulphur point
c)
Triple point of water
d)
Normal boiling point of water
|
|
Rhea Reddy answered |
The triple point of water has a unique value of 273.16 K. At particular value of volume and pressure the triple point of water is always 273.16 K. The melting point of ice and boiling point of water do not have particular values because these points depend on pressure and temperature
While working between temperatures 150 K and 300 K, the entropy change experienced by the Carnot engine during heat addition is 1 kJ/K, the work produced (kJ) by the engine is ________.
- a)100
- b)150
- c)300
- d)600
Correct answer is option 'B'. Can you explain this answer?
While working between temperatures 150 K and 300 K, the entropy change experienced by the Carnot engine during heat addition is 1 kJ/K, the work produced (kJ) by the engine is ________.
a)
100
b)
150
c)
300
d)
600

|
Bhargavi Sengupta answered |
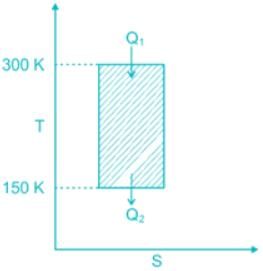
W = Q1 – Q2 = T1ΔS – T2ΔS = (T1 – T2) ΔS = (300 – 150) × 1 = 150 kJ
Two reversible heat engines operating between temperatures 2000 K and T K and T K and 500 K respectively. What is the intermediate temperature, if the efficiency of both the cycles is same?- a)900 K
- b)1000 K
- c)1500 K
- d)1600 K
Correct answer is option 'A'. Can you explain this answer?
Two reversible heat engines operating between temperatures 2000 K and T K and T K and 500 K respectively. What is the intermediate temperature, if the efficiency of both the cycles is same?
a)
900 K
b)
1000 K
c)
1500 K
d)
1600 K
|
|
Tanvi Shah answered |
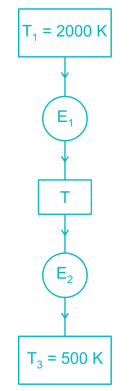
Efficiency of first engine (E1), 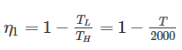
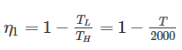
Efficiency of second engine 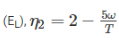
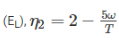
As efficiency of both engine is same,
So, η1 = η2
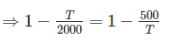
⇒ T2 = 2000 × 50

Short Trick:
When efficiency of both engines are equal, then intermediate temperature
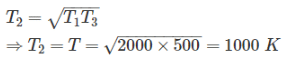
A heat reservoir is maintained at 927 °C. If the ambient temperature is 27 °C, the availability of heat from the reservoir is limited to- a)57%
- b)66%
- c)75%
- d)88%
Correct answer is option 'C'. Can you explain this answer?
A heat reservoir is maintained at 927 °C. If the ambient temperature is 27 °C, the availability of heat from the reservoir is limited to
a)
57%
b)
66%
c)
75%
d)
88%

|
Sreemoyee Deshpande answered |
Availability is the maximum useful work that can be extracted from the system working between two reservoirs.
Availability of Heat = 

Two reversible engines are connected in series between a heat source and a sink. The efficiencies of these engines are 60% and 50%, respectively. If these two engines are replaced by a single reversible engine, the efficiency of this engine will be- a)60%
- b)70%
- c)80%
- d)90%
Correct answer is option 'C'. Can you explain this answer?
Two reversible engines are connected in series between a heat source and a sink. The efficiencies of these engines are 60% and 50%, respectively. If these two engines are replaced by a single reversible engine, the efficiency of this engine will be
a)
60%
b)
70%
c)
80%
d)
90%

|
Bhargavi Sarkar answered |
Let we supply 1 kJ of hat to engine 1 and the rejected heat of engine 1 will go to engine 2.
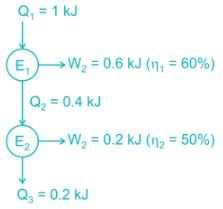
Now, if both engines are replaced by a single engine. So,
∴Efficiencyofnewengine=1−0.21=0.8=80%∴Efficiencyofnewengine=1−0.21=0.8=80%
Short trick: -
ηoverall = η1 + η2 - η1η2
= 0.6 + 0.5 - 0.6 × 0.5 = 0.8 = 80%
A thermometer works on the principle of- a)Law of stable equilibrium
- b)Zeroth law of thermodynamics
- c)First law of thermodynamics
- d)Second law of thermodynamics
Correct answer is option 'B'. Can you explain this answer?
A thermometer works on the principle of
a)
Law of stable equilibrium
b)
Zeroth law of thermodynamics
c)
First law of thermodynamics
d)
Second law of thermodynamics

|
Navya Kaur answered |
A thermometer works on the principle of Zeroth law of Thermodynamics.
According to Zeroth Law of thermodynamics, if two bodies are in thermal equilibrium with a third body then the two bodies are also in thermal equilibrium with each other.
Thermometer are based on the principle of finding the temperature by measuring the thermometric property.
Which one of the following is extensive property of a thermodynamics system- a)Volume
- b)Pressure
- c)Temperature
- d)Density
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is extensive property of a thermodynamics system
a)
Volume
b)
Pressure
c)
Temperature
d)
Density
|
|
Neha Joshi answered |
Intensive Property: These are the properties of system which are independent of mass under consideration. For e.g. Pressure, Temperature, density
Extensive Properties: The properties which depend on the mass of system under consideration.
For e.g Internal Energy, Enthalpy, Volume, Entropy
Note: All specific properties are intensive properties. For e.g. specific volume, specific entropy etc.
Since volume depends on mass hence it is extensive property.
The heat rejection takes place in Carnot cycle at _______.- a)isentropic compression
- b)isentropic expansion
- c)isothermal compression
- d)isothermal expansion
Correct answer is option 'C'. Can you explain this answer?
The heat rejection takes place in Carnot cycle at _______.
a)
isentropic compression
b)
isentropic expansion
c)
isothermal compression
d)
isothermal expansion

|
Rahul Chatterjee answered |
Isothermal compression is compression at constant temperature. It is not trivial because liquids are hardly compressible and gases heat up during compression. Gases heat up during relatively fast compression because the pressure work done onto the gas increases its internal energy and therefore its temperature.
A process in which no heat is supplied or rejected from the system and entropy is not constant is known as- a)Isothermal
- b)Isentropic
- c)Polytropic
- d)Hyperbolic
Correct answer is option 'D'. Can you explain this answer?
A process in which no heat is supplied or rejected from the system and entropy is not constant is known as
a)
Isothermal
b)
Isentropic
c)
Polytropic
d)
Hyperbolic

|
Bijoy Kapoor answered |
Hyperbolic is an adjective describing something that resembles or pertains to a hyperbola (a curve), to hyperbole (an overstatement or exaggeration), or to hyperbolic geometry.
The universal gas constant of a gas is the product of molecular weight of the gas and- a)Gas constant
- b)Specific heat at constant pressure
- c)Specific heat at constant volume
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The universal gas constant of a gas is the product of molecular weight of the gas and
a)
Gas constant
b)
Specific heat at constant pressure
c)
Specific heat at constant volume
d)
None of the above

|
Tanishq Rane answered |
R = Universal Gas Constant
Gas Constant = R/M
⇒ R = Gas constant × Molecular weight.
Which of the following devices complies with the Clausius statement of the second law of thermodynamics?- a)Closed-cycle gas turbine
- b)Internal combustion engine
- c)Steam power plant
- d)Domestic refrigerator
Correct answer is option 'D'. Can you explain this answer?
Which of the following devices complies with the Clausius statement of the second law of thermodynamics?
a)
Closed-cycle gas turbine
b)
Internal combustion engine
c)
Steam power plant
d)
Domestic refrigerator
|
|
Sagarika Dey answered |
Domestic Refrigerator
Domestic refrigerators comply with the Clausius statement of the second law of thermodynamics.
- Clausius Statement: Heat cannot flow from a colder body to a hotter body without the input of work.
- Explanation: A refrigerator operates by removing heat from the inside (colder body) and transferring it to the outside (hotter body) using work input. This process is in accordance with the Clausius statement as the heat is moved from a colder region to a hotter region with the help of a compressor and refrigerant.
- Working Principle: The refrigerant in the evaporator absorbs heat from the inside of the refrigerator, causing it to vaporize and cool the interior. The compressor then compresses the vaporized refrigerant, raising its temperature and pressure. The hot refrigerant releases heat to the surroundings through the condenser coils, condenses back into a liquid, and the cycle repeats.
- Efficiency: The efficiency of a refrigerator is determined by its coefficient of performance (COP), which is the ratio of heat extracted to the work input. A higher COP indicates better efficiency in transferring heat from a colder region to a hotter region.
- Application: Domestic refrigerators, air conditioners, and heat pumps all utilize the principles of the second law of thermodynamics to transfer heat against the natural flow of heat from hot to cold regions.
In conclusion, domestic refrigerators are devices that comply with the Clausius statement of the second law of thermodynamics by efficiently transferring heat from a colder body to a hotter body with the input of work.
Domestic refrigerators comply with the Clausius statement of the second law of thermodynamics.
- Clausius Statement: Heat cannot flow from a colder body to a hotter body without the input of work.
- Explanation: A refrigerator operates by removing heat from the inside (colder body) and transferring it to the outside (hotter body) using work input. This process is in accordance with the Clausius statement as the heat is moved from a colder region to a hotter region with the help of a compressor and refrigerant.
- Working Principle: The refrigerant in the evaporator absorbs heat from the inside of the refrigerator, causing it to vaporize and cool the interior. The compressor then compresses the vaporized refrigerant, raising its temperature and pressure. The hot refrigerant releases heat to the surroundings through the condenser coils, condenses back into a liquid, and the cycle repeats.
- Efficiency: The efficiency of a refrigerator is determined by its coefficient of performance (COP), which is the ratio of heat extracted to the work input. A higher COP indicates better efficiency in transferring heat from a colder region to a hotter region.
- Application: Domestic refrigerators, air conditioners, and heat pumps all utilize the principles of the second law of thermodynamics to transfer heat against the natural flow of heat from hot to cold regions.
In conclusion, domestic refrigerators are devices that comply with the Clausius statement of the second law of thermodynamics by efficiently transferring heat from a colder body to a hotter body with the input of work.
A tank containing air is stirred by a paddle wheel. The work input to the paddle wheel is 9000 kJ and heat transferred to the surroundings from the tank is 3000 kJ. The external work done by the system is:- a)Zero
- b)3000 KJ
- c)6000 KJ
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
A tank containing air is stirred by a paddle wheel. The work input to the paddle wheel is 9000 kJ and heat transferred to the surroundings from the tank is 3000 kJ. The external work done by the system is:
a)
Zero
b)
3000 KJ
c)
6000 KJ
d)
None of these

|
Yash Joshi answered |
First law of thermodynamics:
δQ=ΔU+δWδQ=ΔU+δW
(-3000) = ΔU + (-9000)
ΔU = 6000 kJ
As there is 9000 kJ work done on the system, no work will be done by the system. Energy will be stored in the system as internal energy.
Which of the following properties are NOT sufficient to determine the properties of a vapour? - Temperature
- Pressure
- Dryness fraction
- Specific volume
- a)1 and 2
- b)2 and 3
- c)3 and 4
- d)1 and 4
Correct answer is option 'A'. Can you explain this answer?
Which of the following properties are NOT sufficient to determine the properties of a vapour?
- Temperature
- Pressure
- Dryness fraction
- Specific volume
a)
1 and 2
b)
2 and 3
c)
3 and 4
d)
1 and 4
|
|
Samarth Chaudhary answered |
In properties of vapour, we require at least two independent properties to determine the state of the vapour. Both pressure and temperature are dependent properties so, if we know only pressure and temperature, we can’t determine the properties of vapour.
A heat engine is supplied with 280 kJ/s of heat at a constant fixed temperature of 520 K and heat rejection takes place at 260 K temperature. If the engine is reversible, the heat rejected would be approximate equal to:- a)85 kJ/s
- b)110 kJ/s
- c)140 kJ/s
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
A heat engine is supplied with 280 kJ/s of heat at a constant fixed temperature of 520 K and heat rejection takes place at 260 K temperature. If the engine is reversible, the heat rejected would be approximate equal to:
a)
85 kJ/s
b)
110 kJ/s
c)
140 kJ/s
d)
None of these

|
Srestha Datta answered |
For reversible engine:
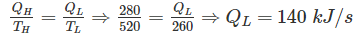
There is fixed mass and energy in- a)An open system
- b)A thermodynamic system
- c)A closed system
- d)An isolated system
Correct answer is option 'D'. Can you explain this answer?
There is fixed mass and energy in
a)
An open system
b)
A thermodynamic system
c)
A closed system
d)
An isolated system
|
|
Anjali Shah answered |
Closed System: There is no mass transfer across the system boundary but energy transfer takes place into or out of the system.
Open System: In this type of system both mass and energy transfer takes place across the boundary of system.
Isolated System: In this type of system neither mass nor energy crosses the boundary of the system. So it is a system with fixed mass and fixed energy.
Dynamic viscosity of most of the gases with rise in temperature...- a)Increases
- b)Decreases
- c)Remains unaffected
- d)Unpredictable
Correct answer is option 'A'. Can you explain this answer?
Dynamic viscosity of most of the gases with rise in temperature...
a)
Increases
b)
Decreases
c)
Remains unaffected
d)
Unpredictable
|
|
Aman Ghosh answered |
Understanding Dynamic Viscosity in Gases
Dynamic viscosity refers to a fluid's internal resistance to flow. In gases, this property is significantly influenced by temperature.
Temperature's Effect on Gas Viscosity
- As temperature increases, the kinetic energy of gas molecules also rises.
- Higher kinetic energy causes gas molecules to move more rapidly and collide with one another more frequently.
Increased Molecular Activity
- With increased molecular motion, the chances of intermolecular collisions grow.
- This results in greater resistance to flow, hence increasing the dynamic viscosity of the gas.
Intermolecular Forces
- Although gases have weaker intermolecular forces compared to liquids, these forces still play a role.
- At elevated temperatures, the energy can overcome some of these forces, but the net effect is a rise in viscosity due to enhanced molecular activity.
Comparison with Liquids
- Unlike gases, the dynamic viscosity of liquids typically decreases with an increase in temperature due to reduced intermolecular attraction.
- This contrast highlights the unique behavior of gases with temperature variations.
Conclusion
- In summary, the correct answer is option 'A': the dynamic viscosity of most gases increases with rising temperature.
- Understanding this relationship is crucial in applications such as fluid dynamics, thermodynamics, and various engineering processes.
Dynamic viscosity refers to a fluid's internal resistance to flow. In gases, this property is significantly influenced by temperature.
Temperature's Effect on Gas Viscosity
- As temperature increases, the kinetic energy of gas molecules also rises.
- Higher kinetic energy causes gas molecules to move more rapidly and collide with one another more frequently.
Increased Molecular Activity
- With increased molecular motion, the chances of intermolecular collisions grow.
- This results in greater resistance to flow, hence increasing the dynamic viscosity of the gas.
Intermolecular Forces
- Although gases have weaker intermolecular forces compared to liquids, these forces still play a role.
- At elevated temperatures, the energy can overcome some of these forces, but the net effect is a rise in viscosity due to enhanced molecular activity.
Comparison with Liquids
- Unlike gases, the dynamic viscosity of liquids typically decreases with an increase in temperature due to reduced intermolecular attraction.
- This contrast highlights the unique behavior of gases with temperature variations.
Conclusion
- In summary, the correct answer is option 'A': the dynamic viscosity of most gases increases with rising temperature.
- Understanding this relationship is crucial in applications such as fluid dynamics, thermodynamics, and various engineering processes.
At critical temperature the _______.- a)Liquid and solid phase can coexist
- b)Liquid and vapour phase can coexist
- c)solid and vapour phase can coexist
- d)Liquid vapour and solid all phase can coexist
Correct answer is option 'B'. Can you explain this answer?
At critical temperature the _______.
a)
Liquid and solid phase can coexist
b)
Liquid and vapour phase can coexist
c)
solid and vapour phase can coexist
d)
Liquid vapour and solid all phase can coexist
|
|
Disha Nambiar answered |
Understanding Critical Temperature
The critical temperature of a substance is a crucial concept in thermodynamics. It represents the temperature above which a substance cannot exist in the liquid state, regardless of the pressure applied.
Key Points about Critical Temperature:
- Definition: The critical temperature is the highest temperature at which a substance can exist as a liquid. Above this temperature, the substance will only exist as a gas, known as vapor.
- Coexistence of Phases: At the critical temperature, the liquid and vapor phases become indistinguishable. This means that the properties of the liquid and vapor phases converge, leading to a single phase known as a supercritical fluid.
- Phase Diagram Insight: In a phase diagram, the critical point marks the end of the liquid-vapor boundary line. Beyond this point, increasing temperature or pressure does not yield distinct liquid or vapor phases.
Why Option B is Correct:
- Liquid and Vapor Coexistence: At the critical temperature, the liquid and vapor phases can coexist in equilibrium. This equilibrium is essential for processes such as distillation and can significantly influence chemical reactions.
- Rejection of Other Options:
- Option A (Liquid and solid phase): This occurs at the melting/freezing point, not at critical temperature.
- Option C (Solid and vapor phase): This is relevant for sublimation but not at critical conditions.
- Option D (All three phases): This is not accurate as the critical temperature pertains specifically to the liquid-vapor coexistence.
Conclusion
In summary, the correct answer is option B since the critical temperature signifies the temperature at which the liquid and vapor phases can coexist, marking a pivotal transition in a substance's phase behavior.
The critical temperature of a substance is a crucial concept in thermodynamics. It represents the temperature above which a substance cannot exist in the liquid state, regardless of the pressure applied.
Key Points about Critical Temperature:
- Definition: The critical temperature is the highest temperature at which a substance can exist as a liquid. Above this temperature, the substance will only exist as a gas, known as vapor.
- Coexistence of Phases: At the critical temperature, the liquid and vapor phases become indistinguishable. This means that the properties of the liquid and vapor phases converge, leading to a single phase known as a supercritical fluid.
- Phase Diagram Insight: In a phase diagram, the critical point marks the end of the liquid-vapor boundary line. Beyond this point, increasing temperature or pressure does not yield distinct liquid or vapor phases.
Why Option B is Correct:
- Liquid and Vapor Coexistence: At the critical temperature, the liquid and vapor phases can coexist in equilibrium. This equilibrium is essential for processes such as distillation and can significantly influence chemical reactions.
- Rejection of Other Options:
- Option A (Liquid and solid phase): This occurs at the melting/freezing point, not at critical temperature.
- Option C (Solid and vapor phase): This is relevant for sublimation but not at critical conditions.
- Option D (All three phases): This is not accurate as the critical temperature pertains specifically to the liquid-vapor coexistence.
Conclusion
In summary, the correct answer is option B since the critical temperature signifies the temperature at which the liquid and vapor phases can coexist, marking a pivotal transition in a substance's phase behavior.
Which gas can attain the highest efficiency for the same compression rise?- a)Any of the gases
- b)Diatomic gases
- c)Mono atomic gases
- d)Tri - atomic gases
Correct answer is option 'C'. Can you explain this answer?
Which gas can attain the highest efficiency for the same compression rise?
a)
Any of the gases
b)
Diatomic gases
c)
Mono atomic gases
d)
Tri - atomic gases

|
Rishika Sen answered |
Monoatomic gases, such as helium and argon, can attain the highest efficiency for the same compression rise compared to other gases. This can be explained by considering the characteristics of monoatomic gases and how they behave under compression.
Monoatomic gases consist of single atoms that do not form bonds with other atoms. This means that the internal energy of monoatomic gases is primarily in the form of kinetic energy associated with the translational motion of the atoms. The atoms move freely and independently of each other.
When a monoatomic gas is compressed, the work done on the gas is primarily used to increase the kinetic energy of the atoms, resulting in an increase in temperature. This is known as an ideal gas law process, where the compression work is directly proportional to the temperature rise.
Now, let's consider the efficiency of the compression process. The efficiency of a gas compression process is defined as the ratio of the work done on the gas to the heat added to the gas. Since the work done on the gas is used to increase the kinetic energy of the atoms, and the heat added to the gas also increases the kinetic energy, the efficiency of the compression process is maximized when the heat added is used most effectively to increase the kinetic energy.
In monoatomic gases, the heat added primarily increases the translational kinetic energy of the atoms, which is the most efficient form of energy storage for these gases. This is because the atoms can freely move and transfer energy through collisions without any additional energy being used to break or form bonds.
On the other hand, diatomic and tri-atomic gases, such as oxygen and nitrogen, have additional internal degrees of freedom, such as rotational and vibrational motion. When these gases are compressed, the heat added not only increases the translational kinetic energy but also distributes some of the energy to the rotational and vibrational modes. This results in a lower efficiency compared to monoatomic gases, as some of the heat energy is used to increase energy in modes other than translational motion.
Therefore, monoatomic gases can attain the highest efficiency for the same compression rise compared to diatomic and tri-atomic gases due to their simpler molecular structure and lack of additional internal degrees of freedom.
Monoatomic gases consist of single atoms that do not form bonds with other atoms. This means that the internal energy of monoatomic gases is primarily in the form of kinetic energy associated with the translational motion of the atoms. The atoms move freely and independently of each other.
When a monoatomic gas is compressed, the work done on the gas is primarily used to increase the kinetic energy of the atoms, resulting in an increase in temperature. This is known as an ideal gas law process, where the compression work is directly proportional to the temperature rise.
Now, let's consider the efficiency of the compression process. The efficiency of a gas compression process is defined as the ratio of the work done on the gas to the heat added to the gas. Since the work done on the gas is used to increase the kinetic energy of the atoms, and the heat added to the gas also increases the kinetic energy, the efficiency of the compression process is maximized when the heat added is used most effectively to increase the kinetic energy.
In monoatomic gases, the heat added primarily increases the translational kinetic energy of the atoms, which is the most efficient form of energy storage for these gases. This is because the atoms can freely move and transfer energy through collisions without any additional energy being used to break or form bonds.
On the other hand, diatomic and tri-atomic gases, such as oxygen and nitrogen, have additional internal degrees of freedom, such as rotational and vibrational motion. When these gases are compressed, the heat added not only increases the translational kinetic energy but also distributes some of the energy to the rotational and vibrational modes. This results in a lower efficiency compared to monoatomic gases, as some of the heat energy is used to increase energy in modes other than translational motion.
Therefore, monoatomic gases can attain the highest efficiency for the same compression rise compared to diatomic and tri-atomic gases due to their simpler molecular structure and lack of additional internal degrees of freedom.
Which of the below stated are properties of a PMM-2? - When the net work is equal to the heat absorbed and work efficiency is 100%.
- Heat is exchanged from one heat reservoir only.
- It violates Kelvin-Planck statement.
- It is a hypothetical machine
- a)1), 2) and 4)
- b)1), 3) and 4)
- c)2), 3) and 4)
- d)1), 2), 3) and 4)
Correct answer is option 'D'. Can you explain this answer?
Which of the below stated are properties of a PMM-2?
- When the net work is equal to the heat absorbed and work efficiency is 100%.
- Heat is exchanged from one heat reservoir only.
- It violates Kelvin-Planck statement.
- It is a hypothetical machine
a)
1), 2) and 4)
b)
1), 3) and 4)
c)
2), 3) and 4)
d)
1), 2), 3) and 4)
|
|
Priyanka Tiwari answered |
Kelvin – Planck statement states “it is impossible to construct an engine, which is operating in a cycle produces no other effect except to external heat from a single reservoir and do equivalent amount of work.
A PMM II is the heat engine which produces the work equal to the heat supplied. Thus, it violates the second law of thermodynamics and It is a hypothetical machine.
On the other hand, Perpetual motion machine of type-I violates the first law of thermodynamics.
In a cyclic process, the work done by the system is 20 kJ, -30 kJ, -5 kJ and 10 kJ. What is the net heat (kJ) for the cyclic process?- a)-5
- b)0
- c)5
- d)10
Correct answer is option 'A'. Can you explain this answer?
In a cyclic process, the work done by the system is 20 kJ, -30 kJ, -5 kJ and 10 kJ. What is the net heat (kJ) for the cyclic process?
a)
-5
b)
0
c)
5
d)
10
|
|
Dhruv Dasgupta answered |
dQ = dW + ΔU (As per first law)
For the cyclic process,
ΔU = 0
∴∫dW=∫dQ∴∫dW=∫dQ
∴ W = 20 - 30 - 5 + 10
= -5 kJ
Which of the following represents the perpetual motion of the first kind- a)engine with 100 % thermal efficiency
- b)a full reversible engine
- c)transfer of heat energy from low temperature source to high temperature source
- d)a machine that continuously creates its own energy
Correct answer is option 'D'. Can you explain this answer?
Which of the following represents the perpetual motion of the first kind
a)
engine with 100 % thermal efficiency
b)
a full reversible engine
c)
transfer of heat energy from low temperature source to high temperature source
d)
a machine that continuously creates its own energy

|
Puja Sharma answered |
The first law of thermodynamics states that the energy can neither be created nor be destroyed. It can only get transformed from one form to another form. An imaginary device which would produce work continuously without absorbing any energy from its surroundings is called a Perpetual Motion Machine of the First kind, (PMMFK). A PMMFK is a device which violates the first law of thermodynamics. It is impossible to devise a PMMFK
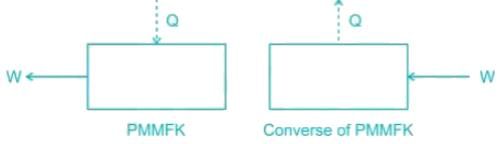
The converse of the above statement is also true, i.e., there can be no machine which would continuously consume work without some other form of energy appearing simultaneously.
A closed bottle containing water at 30°C is carried to the moon in a space-ship. If it is placed on the surface of the moon, what will happen to the water as soon as the lid is opened - a)Water will boil
- b)Water will freeze
- c)Nothing will happen on it
- d)It will decompose into H2 and O2
Correct answer is option 'A'. Can you explain this answer?
A closed bottle containing water at 30°C is carried to the moon in a space-ship. If it is placed on the surface of the moon, what will happen to the water as soon as the lid is opened
a)
Water will boil
b)
Water will freeze
c)
Nothing will happen on it
d)
It will decompose into H2 and O2

|
Aniket Ghoshal answered |
Celsius is placed in a freezer at -10 degrees Celsius. As the water cools down, its temperature decreases until it reaches the freezing point of water, which is 0 degrees Celsius. At this point, the water starts to freeze and turns into ice. The ice will continue to form until all the water is completely frozen. The bottle will contain a mixture of ice and water at 0 degrees Celsius.
An automobile heats up while lying in a parking lot on a sunny day. The process can be assumed to be- a)Isothermal
- b)Isobaric
- c)Isometric
- d)Isentropic
Correct answer is option 'B'. Can you explain this answer?
An automobile heats up while lying in a parking lot on a sunny day. The process can be assumed to be
a)
Isothermal
b)
Isobaric
c)
Isometric
d)
Isentropic
|
|
Sagnik Choudhary answered |
The process of heating up of an automobile can be assumed as isobaric. Because the pressure inside the car is constant. So out of given options the option b is correct.
According to first law of thermodynamics- a)Mass and energy are mutually convertible
- b)Heat and work are mutually convertible
- c)Heat flows from hot substance to cold substance
- d)Carnot engine is most efficient
Correct answer is option 'B'. Can you explain this answer?
According to first law of thermodynamics
a)
Mass and energy are mutually convertible
b)
Heat and work are mutually convertible
c)
Heat flows from hot substance to cold substance
d)
Carnot engine is most efficient
|
|
Isha Nambiar answered |
According to first Law of thermodynamics, “For a closed system undergoing a cycle, net heat transfer is equal to network transfer.”
ΣQ = ΣW
So, heat and work are mutually convertible.
A series of operations, which takes place in a certain order and restore the initial conditions at the end, is known as- a)Reversible cycle
- b)Irreversible cycle
- c)Thermodynamic cycle
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
A series of operations, which takes place in a certain order and restore the initial conditions at the end, is known as
a)
Reversible cycle
b)
Irreversible cycle
c)
Thermodynamic cycle
d)
None of these
|
|
Manoj Pillai answered |
Correct Answer :- c
Explanation : A series of operations, which takes place in a certain order and restores the initial conditions at the end, is known as thermodynamic cycle.
The property of a working substance, which increases or decreases according to the heat supplied or removed in a reversible manner, is called ________.- a)Enthalpy
- b)Entropy
- c)Reversibility
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
The property of a working substance, which increases or decreases according to the heat supplied or removed in a reversible manner, is called ________.
a)
Enthalpy
b)
Entropy
c)
Reversibility
d)
None of these
|
|
Sandeep Sengupta answered |
Entropy
Entropy is the property of a working substance that increases or decreases according to the heat supplied or removed in a reversible manner. It is a measure of the disorder or randomness of a system. In thermodynamics, entropy is denoted by the symbol "S."
Explanation:
Entropy is a fundamental concept in thermodynamics that helps us understand the behavior of energy and heat transfer in a system. It is related to the second law of thermodynamics, which states that the entropy of an isolated system always increases over time.
When heat is supplied to a substance, the energy is transferred to the particles of the substance, increasing their kinetic energy and causing them to move more randomly. This randomness or disorder is reflected in the increase in entropy.
On the other hand, when heat is removed from a substance, the energy is taken away from the particles, reducing their kinetic energy and causing them to become more ordered. This decrease in randomness is reflected in the decrease in entropy.
Entropy can also be understood as a measure of the number of ways in which the energy of a system can be distributed among its particles. A highly ordered system has low entropy, while a highly disordered system has high entropy.
In a reversible process, the heat transfer occurs in such a way that the system can be returned to its initial state without any net change in entropy. Reversible processes are idealized and do not occur in practice, but they serve as a useful concept for understanding the behavior of real processes.
Entropy is a state function, meaning that its value depends only on the state of the system and not on the path taken to reach that state. It can be calculated using thermodynamic relations and equations, such as the Clausius inequality and the Gibbs equation.
In summary, the property of a working substance that increases or decreases according to the heat supplied or removed in a reversible manner is called entropy. It is a measure of the disorder or randomness of a system and plays a crucial role in understanding thermodynamic processes and the second law of thermodynamics.
Entropy is the property of a working substance that increases or decreases according to the heat supplied or removed in a reversible manner. It is a measure of the disorder or randomness of a system. In thermodynamics, entropy is denoted by the symbol "S."
Explanation:
Entropy is a fundamental concept in thermodynamics that helps us understand the behavior of energy and heat transfer in a system. It is related to the second law of thermodynamics, which states that the entropy of an isolated system always increases over time.
When heat is supplied to a substance, the energy is transferred to the particles of the substance, increasing their kinetic energy and causing them to move more randomly. This randomness or disorder is reflected in the increase in entropy.
On the other hand, when heat is removed from a substance, the energy is taken away from the particles, reducing their kinetic energy and causing them to become more ordered. This decrease in randomness is reflected in the decrease in entropy.
Entropy can also be understood as a measure of the number of ways in which the energy of a system can be distributed among its particles. A highly ordered system has low entropy, while a highly disordered system has high entropy.
In a reversible process, the heat transfer occurs in such a way that the system can be returned to its initial state without any net change in entropy. Reversible processes are idealized and do not occur in practice, but they serve as a useful concept for understanding the behavior of real processes.
Entropy is a state function, meaning that its value depends only on the state of the system and not on the path taken to reach that state. It can be calculated using thermodynamic relations and equations, such as the Clausius inequality and the Gibbs equation.
In summary, the property of a working substance that increases or decreases according to the heat supplied or removed in a reversible manner is called entropy. It is a measure of the disorder or randomness of a system and plays a crucial role in understanding thermodynamic processes and the second law of thermodynamics.
The entropy of water at 0 K is assumed to be- a)1 J/K
- b)-1 J/K
- c)10 J/K
- d)0 J/K
Correct answer is option 'D'. Can you explain this answer?
The entropy of water at 0 K is assumed to be
a)
1 J/K
b)
-1 J/K
c)
10 J/K
d)
0 J/K

|
Pankaj Kapoor answered |
Absolute zero (0 K) is a state at which the enthalpy and entropy of a system reach their minimum value, taken as 0.
In mathematical term,
S = k ln W
Where, S is entropy
k is Boltzmann constant
W is thermodynamic probability
When W = 1, it represents the greatest order, S = 0. This occurs only at T = 0 K.
This statement is also known as Third law of thermodynamics.
An isothermal process is governed by- a)Boyle’s law
- b)Charles’ law
- c)Reynold’s law
- d)Nobel’s law
Correct answer is option 'A'. Can you explain this answer?
An isothermal process is governed by
a)
Boyle’s law
b)
Charles’ law
c)
Reynold’s law
d)
Nobel’s law

|
Rishika Sen answered |
's Law
b) Charles's Law
c) Gay-Lussac's Law
d) Avogadro's Law
b) Charles's Law
c) Gay-Lussac's Law
d) Avogadro's Law
Three states of matter are distinguished with respect to molecules by the ______.- a)Atoms in molecules
- b)Number
- c)Orientation
- d)Character of motion
Correct answer is option 'D'. Can you explain this answer?
Three states of matter are distinguished with respect to molecules by the ______.
a)
Atoms in molecules
b)
Number
c)
Orientation
d)
Character of motion
|
|
Anshul Sharma answered |
Matter around us exists in three different states – solid, liquid and gas. They are distinguished by their intermolecular attraction force, molecular arrangement and the mobility of the system.
Particles in a:
- Gas vibrate and move freely at high speeds.
- Liquid vibrate, move about, and slide past each other.
- Solid vibrate (jiggle) but generally do not move from place to place.
The triple point on a T-s diagram is ________.- a)a line
- b)a point
- c)a triangle
- d)not present
Correct answer is option 'A'. Can you explain this answer?
The triple point on a T-s diagram is ________.
a)
a line
b)
a point
c)
a triangle
d)
not present
|
|
Kritika Joshi answered |
Triple Point on a T-s Diagram
The triple point on a T-s (temperature-entropy) diagram is represented by a line, not a point. This is because the triple point is a unique condition where all three phases of a substance (solid, liquid, and gas) coexist in thermodynamic equilibrium. Let's discuss this in more detail.
Definition of Triple Point:
The triple point is a specific combination of temperature and pressure at which a substance can exist in equilibrium in all three phases. At this point, the solid, liquid, and gas phases of the substance can coexist and are in a state of thermodynamic equilibrium.
Representation on a T-s Diagram:
A T-s diagram is a graphical representation of the thermodynamic properties of a substance as it undergoes a change in temperature and entropy. On this diagram, temperature is plotted on the vertical axis (T) and entropy is plotted on the horizontal axis (s).
At the triple point, the substance can exist in all three phases simultaneously. However, the specific values of temperature and entropy at the triple point depend on the substance being considered. For example, the triple point of water occurs at a temperature of 0.01°C and a pressure of 611.657 pascals (Pa).
The line representing the triple point on a T-s diagram is known as the triple line. It separates the regions where two phases coexist from the region where all three phases coexist. The slope of the triple line represents the ratio of the heat of vaporization to the heat of fusion for the substance.
Importance of Triple Point:
The triple point is a crucial reference point in thermodynamics because it provides a fixed and reproducible condition for temperature and pressure. It is often used as a reference in the calibration of temperature scales and pressure measurements.
Conclusion:
In conclusion, the triple point on a T-s diagram is represented by a line, not a point. It is a unique condition where all three phases of a substance coexist in thermodynamic equilibrium. The triple point is important for calibration purposes and is represented by the triple line on a T-s diagram.
The triple point on a T-s (temperature-entropy) diagram is represented by a line, not a point. This is because the triple point is a unique condition where all three phases of a substance (solid, liquid, and gas) coexist in thermodynamic equilibrium. Let's discuss this in more detail.
Definition of Triple Point:
The triple point is a specific combination of temperature and pressure at which a substance can exist in equilibrium in all three phases. At this point, the solid, liquid, and gas phases of the substance can coexist and are in a state of thermodynamic equilibrium.
Representation on a T-s Diagram:
A T-s diagram is a graphical representation of the thermodynamic properties of a substance as it undergoes a change in temperature and entropy. On this diagram, temperature is plotted on the vertical axis (T) and entropy is plotted on the horizontal axis (s).
At the triple point, the substance can exist in all three phases simultaneously. However, the specific values of temperature and entropy at the triple point depend on the substance being considered. For example, the triple point of water occurs at a temperature of 0.01°C and a pressure of 611.657 pascals (Pa).
The line representing the triple point on a T-s diagram is known as the triple line. It separates the regions where two phases coexist from the region where all three phases coexist. The slope of the triple line represents the ratio of the heat of vaporization to the heat of fusion for the substance.
Importance of Triple Point:
The triple point is a crucial reference point in thermodynamics because it provides a fixed and reproducible condition for temperature and pressure. It is often used as a reference in the calibration of temperature scales and pressure measurements.
Conclusion:
In conclusion, the triple point on a T-s diagram is represented by a line, not a point. It is a unique condition where all three phases of a substance coexist in thermodynamic equilibrium. The triple point is important for calibration purposes and is represented by the triple line on a T-s diagram.
In a general compression process, 2 kJ of mechanical work is supplied to 4 kg of fluid and 800 J of heat is rejected to the cooling jacket. The change in specific internal energy would be- a)100 K/kg
- b)1200 J/kg
- c)300 J/kg
- d)400 J/kg
Correct answer is option 'C'. Can you explain this answer?
In a general compression process, 2 kJ of mechanical work is supplied to 4 kg of fluid and 800 J of heat is rejected to the cooling jacket. The change in specific internal energy would be
a)
100 K/kg
b)
1200 J/kg
c)
300 J/kg
d)
400 J/kg
|
|
Avik Ghosh answered |
From the first law of thermodynamics
dQ = dU + dW
dQ = -800 J (As the heat is rejected from the system)
dW = -2000 J (as work is supplied to system)
Change in internal energy = dQ – dW = -800 – (-2000) = 1200 J
Change in specific internal energy = 1200/4 = 300 J/kg (mass is 4 kg)
The property of a working system which changes as the heat is supplied to the working fluid in a reversible manner is known as ________.- a)Entropy
- b)Enthalpy
- c)External energy
- d)Internal energy
Correct answer is option 'A'. Can you explain this answer?
The property of a working system which changes as the heat is supplied to the working fluid in a reversible manner is known as ________.
a)
Entropy
b)
Enthalpy
c)
External energy
d)
Internal energy

|
Alok Iyer answered |
Entropy is a property which is a measure of energy dispersion in a system or the irreversibility. Entropy transfer is associated with heat transfer. If the heat is added to the system, then its entropy increases and if heat is lost from the system, its entropy decreases.
The triple point on a P - V diagram is ________.- a)a line
- b)a point
- c)a triangle
- d)not present
Correct answer is option 'A'. Can you explain this answer?
The triple point on a P - V diagram is ________.
a)
a line
b)
a point
c)
a triangle
d)
not present
|
|
Meera Bose answered |
The triple point on a P-V diagram is a line.
Explanation:
A P-V diagram is a graphical representation of the relationship between pressure (P) and volume (V) of a substance. It is commonly used in thermodynamics to illustrate the changes in state of a substance.
The triple point is a unique point on a P-V diagram that represents the conditions at which a substance can exist in equilibrium in all three states: solid, liquid, and gas. At the triple point, the substance coexists in all three phases simultaneously.
Visual representation:
To visualize this, imagine a P-V diagram with pressure on the y-axis and volume on the x-axis. The triple point would appear as a line on this diagram.
Explanation of the triple point:
The triple point is a specific combination of pressure and temperature at which the solid, liquid, and gas phases of a substance can coexist in equilibrium. It is a critical point where all three phase boundaries meet.
At the triple point, the substance has a unique set of pressure and temperature values. Any deviation from these values will cause a phase transition, where the substance will exist in only one or two phases.
For example, water has a triple point at a pressure of 611.657 pascals (0.00604 atmospheres) and a temperature of 0.01 degrees Celsius (32.018 degrees Fahrenheit). At these specific conditions, water can exist as ice, liquid water, or water vapor simultaneously.
Conclusion:
In summary, the triple point on a P-V diagram is a line. It represents the unique combination of pressure and temperature at which a substance can exist in equilibrium in all three phases: solid, liquid, and gas. Deviating from the triple point conditions will result in a phase transition.
Explanation:
A P-V diagram is a graphical representation of the relationship between pressure (P) and volume (V) of a substance. It is commonly used in thermodynamics to illustrate the changes in state of a substance.
The triple point is a unique point on a P-V diagram that represents the conditions at which a substance can exist in equilibrium in all three states: solid, liquid, and gas. At the triple point, the substance coexists in all three phases simultaneously.
Visual representation:
To visualize this, imagine a P-V diagram with pressure on the y-axis and volume on the x-axis. The triple point would appear as a line on this diagram.
Explanation of the triple point:
The triple point is a specific combination of pressure and temperature at which the solid, liquid, and gas phases of a substance can coexist in equilibrium. It is a critical point where all three phase boundaries meet.
At the triple point, the substance has a unique set of pressure and temperature values. Any deviation from these values will cause a phase transition, where the substance will exist in only one or two phases.
For example, water has a triple point at a pressure of 611.657 pascals (0.00604 atmospheres) and a temperature of 0.01 degrees Celsius (32.018 degrees Fahrenheit). At these specific conditions, water can exist as ice, liquid water, or water vapor simultaneously.
Conclusion:
In summary, the triple point on a P-V diagram is a line. It represents the unique combination of pressure and temperature at which a substance can exist in equilibrium in all three phases: solid, liquid, and gas. Deviating from the triple point conditions will result in a phase transition.
A scientist says that the efficiency of his heat engine which operates at source temperature 127°C and sink temperature 27°C is 26%, then- a)It is impossible
- b)It is possible but less probable
- c) It is quite probable
- d)Data are incomplete
Correct answer is option 'A'. Can you explain this answer?
A scientist says that the efficiency of his heat engine which operates at source temperature 127°C and sink temperature 27°C is 26%, then
a)
It is impossible
b)
It is possible but less probable
c)
It is quite probable
d)
Data are incomplete

|
Megha Choudhury answered |
Carnot Engine, a reversible heat engine has maximum efficiency when operating between two temperature limits.
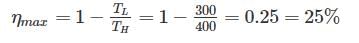
So more the 25% efficiency is not possible.
According to which law, all perfect gases change in volume by (1/273)th of their original volume at 0°C for every 1°C change in temperature when pressure remains constant- a)Joule's law
- b)Boyle's law
- c)Regnault's law
- d)Charles' law
Correct answer is option 'D'. Can you explain this answer?
According to which law, all perfect gases change in volume by (1/273)th of their original volume at 0°C for every 1°C change in temperature when pressure remains constant
a)
Joule's law
b)
Boyle's law
c)
Regnault's law
d)
Charles' law
|
|
Aniket Saini answered |
temperature, when the absolute pressure remains constant." Mathematically,
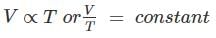
Or “All perfect gases change in volume by 1 / 273 th of its original volume at 0° C for every 1° C change in temperature, when the pressure remains constant."
Boyles law: The absolute pressure of a given mass of a perfect gas varies inversely as its volume, when the temperature remains constant." Mathematically,
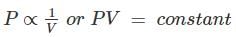
Two gases A and B with their molecular weights 28 and 44 respectively, expand at constant pressures through the same temperature range. The ratio of quantity of work done by the two gases (A : B) is ________.- a)7 : 11
- b)11 : 7
- c)4 : 11
- d)7 : 4
Correct answer is option 'B'. Can you explain this answer?
Two gases A and B with their molecular weights 28 and 44 respectively, expand at constant pressures through the same temperature range. The ratio of quantity of work done by the two gases (A : B) is ________.
a)
7 : 11
b)
11 : 7
c)
4 : 11
d)
7 : 4

|
Manasa Bose answered |
Work done, W = P2V2 - P1V1

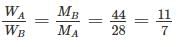
In a surrounding, the amount of irreversibility of a process undergone by a system is determined by ________.- a)entropy change of the system
- b)entropy change of the surrounding
- c)entropy increase of the universe
- d)entropy decrease of the universe
Correct answer is option 'C'. Can you explain this answer?
In a surrounding, the amount of irreversibility of a process undergone by a system is determined by ________.
a)
entropy change of the system
b)
entropy change of the surrounding
c)
entropy increase of the universe
d)
entropy decrease of the universe
|
|
Sagnik Choudhary answered |
The universe is formed by system and surrounding and considered as an isolated system. The entropy of the universe always tends towards a maximum. The entropy increase of an isolated system is a measure of the extent of irreversibility of the process undergone by the system.
If the ratio of the lower to the higher absolute temperature is 7/8, then what will be the COP of the Carnot refrigerator?- a)6
- b)7
- c)8
- d)Insufficient data
Correct answer is option 'B'. Can you explain this answer?
If the ratio of the lower to the higher absolute temperature is 7/8, then what will be the COP of the Carnot refrigerator?
a)
6
b)
7
c)
8
d)
Insufficient data
|
|
Avik Ghosh answered |
The Carnot COP of a refrigerator working between the temperature limit of TH and TL is given as
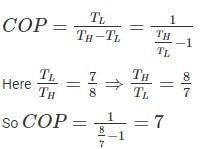
Kindly note that the COP of Carnot heat pump or refrigerator depends only on the upper and lower temperature limits and COP is independent of the working fluid.
A Hot Wire Anemometer is used for the measurement of ______.- a)Pressure of gases
- b)Velocity of gases
- c)Viscosity of gases
- d)Viscosity of liquids
Correct answer is option 'B'. Can you explain this answer?
A Hot Wire Anemometer is used for the measurement of ______.
a)
Pressure of gases
b)
Velocity of gases
c)
Viscosity of gases
d)
Viscosity of liquids
|
|
Kiran Basu answered |
An anemometer is a weather monitor instrument used to measure wind speed.
Basic Principle of Hot wire Anemometer
When an electrically heated wire is placed in a flowing gas stream, heat is transferred from the wire to the gas and hence the temperature of the wire reduces, and due to this, the resistance of the wire also changes. This change in resistance of the wire becomes a measure of flow rate.
Specific heat of a gas, Cp = Cv, at- a)Absolute zero
- b)Critical temperature
- c)Triple point
- d)All temperatures
Correct answer is option 'A'. Can you explain this answer?
Specific heat of a gas, Cp = Cv, at
a)
Absolute zero
b)
Critical temperature
c)
Triple point
d)
All temperatures

|
Aniket Mehta answered |
A. Absolute zero:
At absolute zero temperature (0 Kelvin or -273.15 degrees Celsius), the molecular motion of a gas comes to a complete stop. At this temperature, all gases have zero kinetic energy, and their internal energy is also zero. Therefore, the specific heat at constant volume (Cv) and the specific heat at constant pressure (Cp) of a gas are equal to each other.
B. Critical temperature:
The critical temperature of a substance is the highest temperature at which the gas and liquid phases can coexist in equilibrium. Above this temperature, the substance exists only in the gaseous state. The specific heat of a gas at constant volume (Cv) and constant pressure (Cp) may not be equal at the critical temperature. Therefore, the correct option is A, not B.
C. Triple point:
The triple point of a substance is the unique combination of temperature and pressure at which all three phases (solid, liquid, and gas) can coexist in equilibrium. At the triple point, the specific heat at constant volume (Cv) and constant pressure (Cp) may not be equal. Therefore, the correct option is A, not C.
D. All temperatures:
The specific heat at constant volume (Cv) and constant pressure (Cp) of a gas are generally not equal at all temperatures. The specific heat at constant pressure (Cp) takes into account the heat required to raise the temperature of the gas and also the work done by the gas when it expands against a constant pressure. On the other hand, the specific heat at constant volume (Cv) only considers the heat required to raise the temperature of the gas. Therefore, Cp is always greater than Cv for a gas.
Conclusion:
The specific heat at constant volume (Cv) and constant pressure (Cp) of a gas are equal only at absolute zero temperature (option A). At all other temperatures, Cp is greater than Cv.
At absolute zero temperature (0 Kelvin or -273.15 degrees Celsius), the molecular motion of a gas comes to a complete stop. At this temperature, all gases have zero kinetic energy, and their internal energy is also zero. Therefore, the specific heat at constant volume (Cv) and the specific heat at constant pressure (Cp) of a gas are equal to each other.
B. Critical temperature:
The critical temperature of a substance is the highest temperature at which the gas and liquid phases can coexist in equilibrium. Above this temperature, the substance exists only in the gaseous state. The specific heat of a gas at constant volume (Cv) and constant pressure (Cp) may not be equal at the critical temperature. Therefore, the correct option is A, not B.
C. Triple point:
The triple point of a substance is the unique combination of temperature and pressure at which all three phases (solid, liquid, and gas) can coexist in equilibrium. At the triple point, the specific heat at constant volume (Cv) and constant pressure (Cp) may not be equal. Therefore, the correct option is A, not C.
D. All temperatures:
The specific heat at constant volume (Cv) and constant pressure (Cp) of a gas are generally not equal at all temperatures. The specific heat at constant pressure (Cp) takes into account the heat required to raise the temperature of the gas and also the work done by the gas when it expands against a constant pressure. On the other hand, the specific heat at constant volume (Cv) only considers the heat required to raise the temperature of the gas. Therefore, Cp is always greater than Cv for a gas.
Conclusion:
The specific heat at constant volume (Cv) and constant pressure (Cp) of a gas are equal only at absolute zero temperature (option A). At all other temperatures, Cp is greater than Cv.
Clausius’ statement and Kelvin - Planck’s statement are ________.- a)not connected
- b)two parallel statements of the second law
- c)violation of one does not violates the other
- d)false statements
Correct answer is option 'B'. Can you explain this answer?
Clausius’ statement and Kelvin - Planck’s statement are ________.
a)
not connected
b)
two parallel statements of the second law
c)
violation of one does not violates the other
d)
false statements
|
|
Samarth Chaudhary answered |
There are two statements of second law of thermodynamics.
Clausius statement: No device can operate on a cycle and produce effect that is solely the heat transfer from a lower-temperature body to a higher-temperature body
Kelvin Plank statement: It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work
Equivalence of two statements: If some device violates one statement, it also violates the other statement, and vice versa.
In a Steady flow process,a)heat transfer is constantb)The ratio of work transfer is constantc)The ratio of mass flow at inlet and outlet is samed)All of theseCorrect answer is option 'D'. Can you explain this answer?
|
|
Ayush Chawla answered |
Steady Flow Process
A steady flow process refers to a thermodynamic process in which the properties of a fluid flowing through a system do not change with time. In other words, the conditions of the fluid at any given point within the system remain constant over time.
Heat Transfer
In a steady flow process, heat transfer is constant. This means that the rate of heat transfer into or out of the system remains the same throughout the process. The amount of heat added or removed per unit time is consistent, and there are no fluctuations in the heat transfer rate.
Work Transfer
The ratio of work transfer is constant in a steady flow process. This means that the amount of work done by or on the system per unit time remains the same throughout the process. The work transfer can be positive (work done by the system) or negative (work done on the system), but the ratio remains constant.
Mass Flow
In a steady flow process, the ratio of mass flow at the inlet and outlet is the same. This means that the mass entering the system is equal to the mass leaving the system. The mass flow rate is constant throughout the process, and there are no changes in the amount of mass entering or leaving the system.
Conclusion
In conclusion, all of the given options are correct for a steady flow process. Heat transfer remains constant, the ratio of work transfer is constant, and the ratio of mass flow at the inlet and outlet is the same. These characteristics define a steady flow process and are fundamental principles in thermodynamics.
A steady flow process refers to a thermodynamic process in which the properties of a fluid flowing through a system do not change with time. In other words, the conditions of the fluid at any given point within the system remain constant over time.
Heat Transfer
In a steady flow process, heat transfer is constant. This means that the rate of heat transfer into or out of the system remains the same throughout the process. The amount of heat added or removed per unit time is consistent, and there are no fluctuations in the heat transfer rate.
Work Transfer
The ratio of work transfer is constant in a steady flow process. This means that the amount of work done by or on the system per unit time remains the same throughout the process. The work transfer can be positive (work done by the system) or negative (work done on the system), but the ratio remains constant.
Mass Flow
In a steady flow process, the ratio of mass flow at the inlet and outlet is the same. This means that the mass entering the system is equal to the mass leaving the system. The mass flow rate is constant throughout the process, and there are no changes in the amount of mass entering or leaving the system.
Conclusion
In conclusion, all of the given options are correct for a steady flow process. Heat transfer remains constant, the ratio of work transfer is constant, and the ratio of mass flow at the inlet and outlet is the same. These characteristics define a steady flow process and are fundamental principles in thermodynamics.
Efficiency of a Carnot engine is 75%. If the cycle direction is reverse, COP of the heat pump working on reversed Carnot cycle will be- a)1.33
- b)0.75
- c)0.33
- d)1.75
Correct answer is option 'A'. Can you explain this answer?
Efficiency of a Carnot engine is 75%. If the cycle direction is reverse, COP of the heat pump working on reversed Carnot cycle will be
a)
1.33
b)
0.75
c)
0.33
d)
1.75

|
Sahil Chawla answered |
Efficiency of Carnot cycle 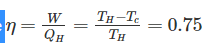
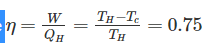
By reversing the Carnot cycle we can have either heat pump or refrigerator.
COP of heat pump = 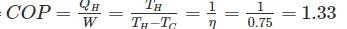
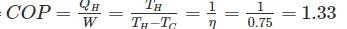
If the entropy of the universe decreases. What this depicts about the nature of the process?- a)Ideal process
- b)Reversible process
- c)Irreversible process
- d)Impossible process
Correct answer is option 'D'. Can you explain this answer?
If the entropy of the universe decreases. What this depicts about the nature of the process?
a)
Ideal process
b)
Reversible process
c)
Irreversible process
d)
Impossible process
|
|
Sharmila Chauhan answered |
Explanation:
Entropy is a measure of the disorder or randomness in a system. The Second Law of Thermodynamics states that the total entropy of an isolated system always increases over time. Therefore, if the entropy of the universe decreases, it would violate this fundamental law and be considered an impossible process.
Understanding the Options:
Let's analyze the other options to understand why they are not correct in this scenario:
a) Ideal Process: An ideal process is one that occurs without any losses or inefficiencies. It is characterized by reversible changes, and the entropy of the system remains constant. However, the question states that the entropy of the universe decreases, which is not possible in an ideal process.
b) Reversible Process: A reversible process is one that can be reversed by an infinitesimal change in the system's conditions. In a reversible process, the system and its surroundings can be brought back to their initial state without any net entropy change. Since the question states that the entropy of the universe decreases, it contradicts the characteristics of a reversible process.
c) Irreversible Process: An irreversible process is one that occurs with losses or irreversibilities. It is characterized by an increase in the entropy of the system and the surroundings. The entropy of the universe always increases in an irreversible process, so option (c) is also incorrect.
Conclusion:
Based on the Second Law of Thermodynamics, the entropy of the universe always increases or remains constant. If the entropy of the universe were to decrease, it would violate this fundamental law and be considered an impossible process. Therefore, option (d) is the correct answer.
Entropy is a measure of the disorder or randomness in a system. The Second Law of Thermodynamics states that the total entropy of an isolated system always increases over time. Therefore, if the entropy of the universe decreases, it would violate this fundamental law and be considered an impossible process.
Understanding the Options:
Let's analyze the other options to understand why they are not correct in this scenario:
a) Ideal Process: An ideal process is one that occurs without any losses or inefficiencies. It is characterized by reversible changes, and the entropy of the system remains constant. However, the question states that the entropy of the universe decreases, which is not possible in an ideal process.
b) Reversible Process: A reversible process is one that can be reversed by an infinitesimal change in the system's conditions. In a reversible process, the system and its surroundings can be brought back to their initial state without any net entropy change. Since the question states that the entropy of the universe decreases, it contradicts the characteristics of a reversible process.
c) Irreversible Process: An irreversible process is one that occurs with losses or irreversibilities. It is characterized by an increase in the entropy of the system and the surroundings. The entropy of the universe always increases in an irreversible process, so option (c) is also incorrect.
Conclusion:
Based on the Second Law of Thermodynamics, the entropy of the universe always increases or remains constant. If the entropy of the universe were to decrease, it would violate this fundamental law and be considered an impossible process. Therefore, option (d) is the correct answer.
An inventor says, he has invented an engine which will reject 20% of heat absorbed from the source and the engine operates between 2000 K and 500 K. What kind of engine it is?- a)Carnot engine
- b)Diesel engine
- c)Dual engine
- d)Impossible engine
Correct answer is option 'D'. Can you explain this answer?
An inventor says, he has invented an engine which will reject 20% of heat absorbed from the source and the engine operates between 2000 K and 500 K. What kind of engine it is?
a)
Carnot engine
b)
Diesel engine
c)
Dual engine
d)
Impossible engine

|
Rithika Kaur answered |
T1 = 2000 K
T2 = 500 K
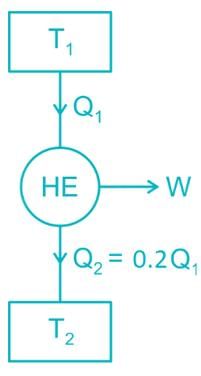
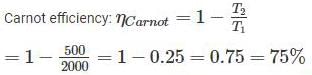
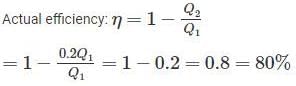
Since, the actual efficiency is more than the Carnot efficiency, so this engine has an impossible cycle.
Carnot cycle consists of- a)Two constant volume and two isentropic processes
- b)Two isothermal and two isentropic processes
- c)Two constant pressure and two isentropic processes
- d)One constant volume, one constant pressure and two isentropic processes
Correct answer is option 'B'. Can you explain this answer?
Carnot cycle consists of
a)
Two constant volume and two isentropic processes
b)
Two isothermal and two isentropic processes
c)
Two constant pressure and two isentropic processes
d)
One constant volume, one constant pressure and two isentropic processes

|
Hiral Sharma answered |
Carnot cycle is one of the best-known reversible cycles. The Carnot cycle is composed of four reversible processes.
- Reversible Isothermal Expansion (process 1-2)
- Reversible adiabatic expansion (process 2-3)
- Reversible isothermal compression (process 3-4)
- Reversible adiabatic compression (process 4-1)
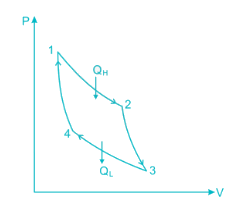
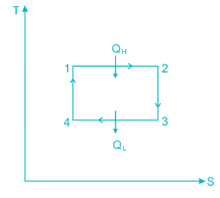 Fig. P-V and T-S diagrams of Carnot Cycle
Fig. P-V and T-S diagrams of Carnot Cycle
Isothermal compressibility of an ideal gas is - a)nR/VP
- b)nRT/VP2
- c)RT/VP2
- d)nRT/VP
Correct answer is option 'B'. Can you explain this answer?
Isothermal compressibility of an ideal gas is
a)
nR/VP
b)
nRT/VP2
c)
RT/VP2
d)
nRT/VP

|
Ishani Basu answered |
Explanation:
The isothermal compressibility (β) of a substance is a measure of how much the volume of the substance changes when the pressure is changed at constant temperature. It is defined as the fractional change in volume per unit change in pressure.
Formula:
The formula for isothermal compressibility is given by:
β = -1/V (∂V/∂P)T
Derivation:
Consider an ideal gas enclosed in a container. The volume (V) of the gas can be expressed in terms of pressure (P) and temperature (T) using the ideal gas law equation:
PV = nRT
Where:
P = pressure of the gas
V = volume of the gas
n = number of moles of the gas
R = universal gas constant
T = temperature of the gas
Step 1: Differentiate the ideal gas law equation with respect to pressure at constant temperature:
∂(PV)/∂P = ∂(nRT)/∂P
V + P (∂V/∂P) = nR (∂T/∂P)
Step 2: Rearrange the equation to isolate (∂V/∂P):
(∂V/∂P) = (nR/V) (∂T/∂P)
(∂V/∂P)T = (nR/V) (∂T/∂P)
Step 3: Substitute the formula for (∂V/∂P)T into the equation for isothermal compressibility:
β = -1/V (∂V/∂P)T
β = -1/V [(nR/V) (∂T/∂P)]
β = -nR/(VP) (∂T/∂P)
Step 4: Simplify the equation by substituting nR/V with 1/T (using the ideal gas law):
β = -1/VP (∂T/∂P)
β = -RT/VP2
Conclusion:
The correct expression for the isothermal compressibility (β) of an ideal gas is -RT/VP2. Therefore, option 'B' is the correct answer.
The isothermal compressibility (β) of a substance is a measure of how much the volume of the substance changes when the pressure is changed at constant temperature. It is defined as the fractional change in volume per unit change in pressure.
Formula:
The formula for isothermal compressibility is given by:
β = -1/V (∂V/∂P)T
Derivation:
Consider an ideal gas enclosed in a container. The volume (V) of the gas can be expressed in terms of pressure (P) and temperature (T) using the ideal gas law equation:
PV = nRT
Where:
P = pressure of the gas
V = volume of the gas
n = number of moles of the gas
R = universal gas constant
T = temperature of the gas
Step 1: Differentiate the ideal gas law equation with respect to pressure at constant temperature:
∂(PV)/∂P = ∂(nRT)/∂P
V + P (∂V/∂P) = nR (∂T/∂P)
Step 2: Rearrange the equation to isolate (∂V/∂P):
(∂V/∂P) = (nR/V) (∂T/∂P)
(∂V/∂P)T = (nR/V) (∂T/∂P)
Step 3: Substitute the formula for (∂V/∂P)T into the equation for isothermal compressibility:
β = -1/V (∂V/∂P)T
β = -1/V [(nR/V) (∂T/∂P)]
β = -nR/(VP) (∂T/∂P)
Step 4: Simplify the equation by substituting nR/V with 1/T (using the ideal gas law):
β = -1/VP (∂T/∂P)
β = -RT/VP2
Conclusion:
The correct expression for the isothermal compressibility (β) of an ideal gas is -RT/VP2. Therefore, option 'B' is the correct answer.
Properties of substances like pressure, temperature and density, in thermodynamic co-ordinates are _____.- a)Path functions
- b)Point functions
- c)Cyclic functions
- d)Real functions
Correct answer is option 'B'. Can you explain this answer?
Properties of substances like pressure, temperature and density, in thermodynamic co-ordinates are _____.
a)
Path functions
b)
Point functions
c)
Cyclic functions
d)
Real functions

|
Ashish Chakraborty answered |
The thermodynamic properties which depends on the end states only (independent of the path followed) are known as point function like temperature, pressure, density, volume, enthalpy, entropy etc.
The thermodynamic properties which depends on the end states as well as the path followed are known as path function like heat and work.
Chapter doubts & questions for Thermodynamics - SSC JE Mechanical Mock Test Series 2026 2025 is part of Mechanical Engineering exam preparation. The chapters have been prepared according to the Mechanical Engineering exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Mechanical Engineering 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Thermodynamics - SSC JE Mechanical Mock Test Series 2026 in English & Hindi are available as part of Mechanical Engineering exam.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
SSC JE Mechanical Mock Test Series 2026
3 videos|1 docs|55 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup