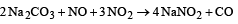All Exams >
NEET >
Chemistry 31 Years NEET Chapterwise Solved Papers >
All Questions
All questions of The s - Block Elements for NEET Exam
The sequence of ioinic mobility in aqueous solution is : [2008]- a)K+ > Na+ > Rb+ > Cs+
- b)Cs+ > Rb+ > K+ > Na+
- c)Rb+ > K+ > Cs+ > Na+
- d)Na+ > K+ > Rb+ > Cs+
Correct answer is option 'B'. Can you explain this answer?
The sequence of ioinic mobility in aqueous solution is : [2008]
a)
K+ > Na+ > Rb+ > Cs+
b)
Cs+ > Rb+ > K+ > Na+
c)
Rb+ > K+ > Cs+ > Na+
d)
Na+ > K+ > Rb+ > Cs+

|
Rohan Unni answered |
Smaller the ion more is its ionic mobility in aqueous solution. Ionic radii of the given alkali metals is in the order Na+ < K+ < Rb+ < Cs+ and thus expected ionic mobility will be in the order Cs+ < Rb+ < K+ < Na+. However due to high degree of solvation (or hydration) because of lower size or high charge density, the hydrated ion size follows the order Cs+ < Rb+ < K+ < Na+ and thus conductivity order is Cs+ > Rb+ > K+ > Na+ i.e. option (b) is correct answer.
Which of the following statement is false ? [1994]- a)Strontium decomposes water readily than beryllium
- b)Barium carbonate melts at a higher temperature than calcium carbonate
- c)Barium hydroxide is more soluble in water than magnesium hydroxide
- d)Beryllium hydroxide is more basic than barium hydroxide.
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is false ? [1994]
a)
Strontium decomposes water readily than beryllium
b)
Barium carbonate melts at a higher temperature than calcium carbonate
c)
Barium hydroxide is more soluble in water than magnesium hydroxide
d)
Beryllium hydroxide is more basic than barium hydroxide.

|
Pankaj Banerjee answered |
Be(OH)2 is amphoteric, but the hydroxides of other alkaline earth metals are basic. The basic strength increases gradually.
In which of the following the hydration energy is higher than the lattice energy? [2007]- a)MgSO4
- b)RaSO4
- c)SrSO4
- d)BaSO4
Correct answer is option 'A'. Can you explain this answer?
In which of the following the hydration energy is higher than the lattice energy? [2007]
a)
MgSO4
b)
RaSO4
c)
SrSO4
d)
BaSO4

|
Pankaj Banerjee answered |
The solubility of sulphates of alkaline earth metals decreases as we move down the group from Be to Ba due to the reason that ionic size increases down the group. The lattice energy remains constant because sulphate ion is so large, so that small change in cationic sizes do not make any difference.
Thus the order:
Thus the order:
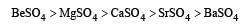
Identify the correct statement [1995]- a)gypsum is obtained by heating plaster of Paris
- b)plaster of Paris can be obtained by hydration of gypsum
- c)plaster of par is is obtain ed by par tial oxidation of gypsum
- d)gypsum contains a lower percentage of calcium than plaster of Paris
Correct answer is option 'D'. Can you explain this answer?
Identify the correct statement [1995]
a)
gypsum is obtained by heating plaster of Paris
b)
plaster of Paris can be obtained by hydration of gypsum
c)
plaster of par is is obtain ed by par tial oxidation of gypsum
d)
gypsum contains a lower percentage of calcium than plaster of Paris

|
Rajeev Sharma answered |
Gypsum is CaSO4. 2H2O and plaster of Paris i s (CaSO4)2 .H2O. Therefore gypsum contains a lower percentage of calcium than plaster of Paris.
Which one of the following has minimum value of cation/anion ratio. [1993]- a)NaCl
- b)KCl
- c)MgCl2
- d)CaF2
Correct answer is option 'C'. Can you explain this answer?
Which one of the following has minimum value of cation/anion ratio. [1993]
a)
NaCl
b)
KCl
c)
MgCl2
d)
CaF2

|
Prasenjit Pillai answered |
Atomic size of K+ > Ca2+ > Mg2+ and that of Cl– > F–. Therefore, Mg2+/Cl– ratio has the minimum value.
In the replacement reaction The reaction will be most favourable if M happens to be : [2012 M]
The reaction will be most favourable if M happens to be : [2012 M]- a)Na
- b)K
- c)Rb
- d)Li
Correct answer is option 'C'. Can you explain this answer?
In the replacement reaction

The reaction will be most favourable if M happens to be : [2012 M]
a)
Na
b)
K
c)
Rb
d)
Li

|
Tejas Chavan answered |
Tertiary halide can show ionic reaction with MF so, MF should be most ionic for reaction to proceed forward. Hence ‘M’ should be ‘Rb’.
Property of the alkaline earth metals that increases with their atomic number : [2010]- a)Solubility of their hydroxides in water
- b)Solubility of their sulphates in water
- c)Ionization energy
- d)Electronegativity
Correct answer is option 'A'. Can you explain this answer?
Property of the alkaline earth metals that increases with their atomic number : [2010]
a)
Solubility of their hydroxides in water
b)
Solubility of their sulphates in water
c)
Ionization energy
d)
Electronegativity

|
Arpita Tiwari answered |
Lattice energy decreases more rapidly than hydration energy for alkaline earth metal hydroxides.
A solid compound ‘X’ on heating gives CO2 gas and a residue. The residue mixed with water forms ‘Y’. On passing an excess of CO2 through ‘Y’ in water, a clear solution ‘Z’, is obtained. On boiling ‘Z’, a compound ‘X’ is reformed. The compound ‘X’ is[2004]- a)Ca(HCO3)2
- b)CaCO3
- c)Na2CO3
- d)K2CO3
Correct answer is option 'B'. Can you explain this answer?
A solid compound ‘X’ on heating gives CO2 gas and a residue. The residue mixed with water forms ‘Y’. On passing an excess of CO2 through ‘Y’ in water, a clear solution ‘Z’, is obtained. On boiling ‘Z’, a compound ‘X’ is reformed. The compound ‘X’ is[2004]
a)
Ca(HCO3)2
b)
CaCO3
c)
Na2CO3
d)
K2CO3

|
Arpita Tiwari answered |
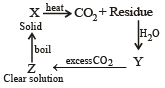
The given properties coincide with CaCO3
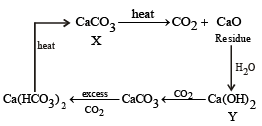
Which of the following is known as fusion mixture? [1994]- a)Mixture of Na2CO3 + NaHCO3
- b)Na2CO3.10H2O
- c)Mixture of K2CO3 + Na2CO3
- d)NaHCO3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is known as fusion mixture? [1994]
a)
Mixture of Na2CO3 + NaHCO3
b)
Na2CO3.10H2O
c)
Mixture of K2CO3 + Na2CO3
d)
NaHCO3

|
Krish Chakraborty answered |
Mixture of K2CO3 and Na2CO3 is called fusion mixture
The correct order of the mobility of the alkali metal ions in aqueous solutions is [2006]- a)Na+ > K+ > Rb+ > Li+
- b)K+ > Rb+ > Na+ > Li+
- c)Rb+ >K+ > Na+ > Li+
- d)Li+ > Na+ > K+ > Rb+
Correct answer is option 'C'. Can you explain this answer?
The correct order of the mobility of the alkali metal ions in aqueous solutions is [2006]
a)
Na+ > K+ > Rb+ > Li+
b)
K+ > Rb+ > Na+ > Li+
c)
Rb+ >K+ > Na+ > Li+
d)
Li+ > Na+ > K+ > Rb+

|
Krish Chakraborty answered |
Ionic radii of alkali metals in water follows the order Li+ > Na+ > K+ > Rb+ > Cs+
Thus in aqueous solution due to larger ionic radius Li+ has lowest mobility and hence the correct order of ionic mobility is Li + < Na + < K + < Rb+
Thus in aqueous solution due to larger ionic radius Li+ has lowest mobility and hence the correct order of ionic mobility is Li + < Na + < K + < Rb+
Compared with the alkaline earth metals, the alkali metals exhibit [1990]- a)Smaller ionic radii
- b)Highest boiling points
- c)Greater hardness
- d)Lower ionization energies.
Correct answer is option 'D'. Can you explain this answer?
Compared with the alkaline earth metals, the alkali metals exhibit [1990]
a)
Smaller ionic radii
b)
Highest boiling points
c)
Greater hardness
d)
Lower ionization energies.

|
Dipanjan Chawla answered |
Because of larger size and smaller nuclear charge, alkali metals have low ionization potential relative to alkaline earth metals.
Which one of the following is present as an active ingredient in bleaching powder for bleaching action ? [2011]- a)CaOCl2
- b)Ca(OCl)2
- c)CaO2Cl
- d)CaCl2
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is present as an active ingredient in bleaching powder for bleaching action ? [2011]
a)
CaOCl2
b)
Ca(OCl)2
c)
CaO2Cl
d)
CaCl2

|
Ritika Khanna answered |
Active ingredient in bleaching powder for bleaching action is Ca (OCl)2
All the following substances react with water.The pair that gives the same gaseous product is [1994]- a)K and KO2
- b)Na and Na2O2
- c)Ca and CaH2
- d)Ba and BaO2
Correct answer is option 'C'. Can you explain this answer?
All the following substances react with water.The pair that gives the same gaseous product is [1994]
a)
K and KO2
b)
Na and Na2O2
c)
Ca and CaH2
d)
Ba and BaO2

|
Palak Khanna answered |
The pair which gives the same gaseous product is Ca and CaH2.
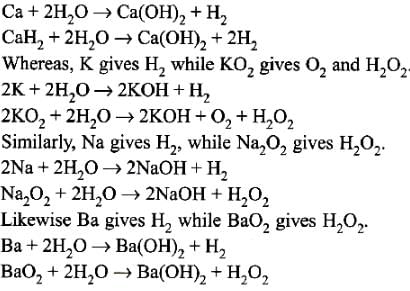
Which one of the following properties of alkali metals increases in magnitude as the atomic number rises ? [1989]- a)Ionic radius
- b)Melting point
- c)Electronegativity
- d)First ionization energy.
Correct answer is option 'A'. Can you explain this answer?
Which one of the following properties of alkali metals increases in magnitude as the atomic number rises ? [1989]
a)
Ionic radius
b)
Melting point
c)
Electronegativity
d)
First ionization energy.
|
|
Priya Deshpande answered |
Alkali metals are a group of elements in the periodic table that includes lithium, sodium, potassium, rubidium, cesium, and francium. These elements have similar properties, such as low electronegativity, high reactivity, and the tendency to lose one electron to form a univalent cation. Among the properties of alkali metals, the ionic radius increases in magnitude as the atomic number rises.
Explanation:
Ionic radius is the size of an ion, which is determined by the number of electrons and protons in the ion. In the case of alkali metals, the valence electron is located in the outermost shell, which means that the ionic radius depends on the size of the outermost shell. As the atomic number increases in the group, the number of electrons and protons also increases, which results in a larger size of the outermost shell. Therefore, the ionic radius increases in magnitude as the atomic number rises.
This trend can be observed in the periodic table by comparing the ionic radii of lithium, sodium, potassium, rubidium, cesium, and francium. The ionic radius increases from lithium to francium due to the increasing number of electrons and protons in the outermost shell.
In summary, the ionic radius of alkali metals increases in magnitude as the atomic number rises. This trend is due to the increasing size of the outermost shell as the number of electrons and protons increases.
Explanation:
Ionic radius is the size of an ion, which is determined by the number of electrons and protons in the ion. In the case of alkali metals, the valence electron is located in the outermost shell, which means that the ionic radius depends on the size of the outermost shell. As the atomic number increases in the group, the number of electrons and protons also increases, which results in a larger size of the outermost shell. Therefore, the ionic radius increases in magnitude as the atomic number rises.
This trend can be observed in the periodic table by comparing the ionic radii of lithium, sodium, potassium, rubidium, cesium, and francium. The ionic radius increases from lithium to francium due to the increasing number of electrons and protons in the outermost shell.
In summary, the ionic radius of alkali metals increases in magnitude as the atomic number rises. This trend is due to the increasing size of the outermost shell as the number of electrons and protons increases.
Which of the following statements is incorrect ? [2011M]- a)Pure sodium metal dissolves in liquid ammonia to give blue solution.
- b)NaOH reacts with glass to give sodium silicate
- c)Aluminium reacts with excess NaOH to give Al(OH)3
- d)NaHCO3 on heating gives Na2CO3
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements is incorrect ? [2011M]
a)
Pure sodium metal dissolves in liquid ammonia to give blue solution.
b)
NaOH reacts with glass to give sodium silicate
c)
Aluminium reacts with excess NaOH to give Al(OH)3
d)
NaHCO3 on heating gives Na2CO3

|
Soumya Ahuja answered |
In crystals of which one of the following ionic compounds would you expect maximum distance between centres of cations and anions? [1998]- a)LiF
- b)CsF
- c)CsI
- d)LiI
Correct answer is option 'C'. Can you explain this answer?
In crystals of which one of the following ionic compounds would you expect maximum distance between centres of cations and anions? [1998]
a)
LiF
b)
CsF
c)
CsI
d)
LiI

|
Deepak Joshi answered |
As Cs+ ion has larger size than Li+ and I– has larger size than F–, therefore maximum distance between centres of cations and anions is in CsI.
Among the following oxides, the one which is most basic is [1994]- a)ZnO
- b)MgO
- c)Al2O3
- d)N2O5
Correct answer is option 'B'. Can you explain this answer?
Among the following oxides, the one which is most basic is [1994]
a)
ZnO
b)
MgO
c)
Al2O3
d)
N2O5

|
Maya Sengupta answered |
MgO. N2O5 is strongly acidic, ZnO and Al2 O3 are amphoteric, therefore, MgO is most basic.
Washing soda has formula [1990]- a)Na2CO3.7H2O
- b)Na2CO3.10H2O
- c)Na2CO3.3H2O
- d)Na2CO3
Correct answer is option 'B'. Can you explain this answer?
Washing soda has formula [1990]
a)
Na2CO3.7H2O
b)
Na2CO3.10H2O
c)
Na2CO3.3H2O
d)
Na2CO3

|
Krish Chakraborty answered |
Washing soda is Na2 CO3. 10 H2O.
Which of the following metal ions plays an important role in muscle contraction ? [1994]- a)K+
- b)Na+
- c)Mg2+
- d)Ca2+
Correct answer is option 'D'. Can you explain this answer?
Which of the following metal ions plays an important role in muscle contraction ? [1994]
a)
K+
b)
Na+
c)
Mg2+
d)
Ca2+

|
Ayush Chavan answered |
Ca2+ ions is an essential element for the contraction of muscles.
Electronic configuration of calcium atom may be written as [1992]- a)Ne, 4p2
- b)Ar, 4s2
- c)Ne, 4s2
- d)Ar, 4p2
Correct answer is option 'B'. Can you explain this answer?
Electronic configuration of calcium atom may be written as [1992]
a)
Ne, 4p2
b)
Ar, 4s2
c)
Ne, 4s2
d)
Ar, 4p2

|
Anand Jain answered |
Ca(20) = 1s22s23p6 3s23p6 4s2 = [Ar] 4s2
In which of the following processes, fused sodium hydroxide is electrolysed at a 330ºC temperature for extraction of sodium? [2000]- a)Castner's process
- b)Down's process
- c)Cyanide process
- d)Both 'b' and 'c'
Correct answer is option 'A'. Can you explain this answer?
In which of the following processes, fused sodium hydroxide is electrolysed at a 330ºC temperature for extraction of sodium? [2000]
a)
Castner's process
b)
Down's process
c)
Cyanide process
d)
Both 'b' and 'c'

|
Rohan Unni answered |
In Castner process, for production of (Na) Sodium metal, Sodium hydroxide (NaOH) is electrolysed at temperature 330ºC.
Wh ich of the following atoms will have the smallest size ? [1989]- a)Mg
- b)Na
- c)Be
- d)Li
Correct answer is option 'C'. Can you explain this answer?
Wh ich of the following atoms will have the smallest size ? [1989]
a)
Mg
b)
Na
c)
Be
d)
Li

|
Ayush Chavan answered |
Within a period, the atomic size decreases from left to right.Further atomic size increases down the group. Hence the correct order is i,e. Na > Mg > Li > Be.
Which of the following has largest size ? [1993]- a)Na
- b)Na+
- c)Na–
- d)Can’t be predicted
Correct answer is option 'C'. Can you explain this answer?
Which of the following has largest size ? [1993]
a)
Na
b)
Na+
c)
Na–
d)
Can’t be predicted

|
Kunal Rane answered |
A cation is always much smaller than the corresponding atom, whereas an anion is always larger than the corresponding atom, hence the size decreases in the order
Na- > Na > Na+
Which of the following compounds has the lowest melting point ? [2011]- a)CaCl2
- b)CaBr2
- c)CaI2
- d)CaF2
Correct answer is option 'C'. Can you explain this answer?
Which of the following compounds has the lowest melting point ? [2011]
a)
CaCl2
b)
CaBr2
c)
CaI2
d)
CaF2

|
Kunal Rane answered |
Melting points of halides decreases as the size of the halogen increases. The correct order is CaF2 > CaCl2 > CaBr2 > CaI2
Which of the following alkaline earth metal sulphates has hydration enthalpy higher than the lattice enthalpy? [2010]- a)CaSO4
- b)BeSO4
- c)BaSO4
- d)SrSO4
Correct answer is option 'B'. Can you explain this answer?
Which of the following alkaline earth metal sulphates has hydration enthalpy higher than the lattice enthalpy? [2010]
a)
CaSO4
b)
BeSO4
c)
BaSO4
d)
SrSO4

|
Anand Jain answered |
Be2+ is very small, hence its hydration enthalpy is greater than its lattice enthalpy (At. Nos. Mn = 25, Fe = 26, Co = 27, Ni = 28)
Match List – I with List –II for the compositions of substances and select the correct answer using the code given below the lists : [2011M]
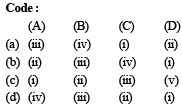
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Match List – I with List –II for the compositions of substances and select the correct answer using the code given below the lists : [2011M]


a)
a
b)
b
c)
c
d)
d

|
Krish Patel answered |
(A) Plaster of paris = 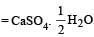
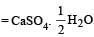
(B) Epsomite = MgSO4.7H2O
(C) Kieserite = MgSO4.H2O
(D) Gypsum = CaSO4.2H2O
(C) Kieserite = MgSO4.H2O
(D) Gypsum = CaSO4.2H2O
In Castner-Kellner cell for production of sodium hydroxide: [NEET Kar. 2013]- a)Brine is electrolyzed with Pt electrodes
- b)Brine is electr olyzed using graphite electrodes
- c)Molten sodium chloride is electrolysed
- d)Sodium amalgam is for med at mercury cathode
Correct answer is option 'D'. Can you explain this answer?
In Castner-Kellner cell for production of sodium hydroxide: [NEET Kar. 2013]
a)
Brine is electrolyzed with Pt electrodes
b)
Brine is electr olyzed using graphite electrodes
c)
Molten sodium chloride is electrolysed
d)
Sodium amalgam is for med at mercury cathode

|
Ruchi Chopra answered |
At the cathode, since the discharge + ions is lower than that of H+ ions on the mercury, cathode, so Na+ ions are discharged while H+ ions remain in the solution.
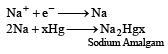
The ease of adsorption of the hydrated alkali metal ions on an ion-exchange resins follows the order :[2012]- a)Li+ < K+ < Na+ < Rb+
- b)Rb+ < K+ <Na+ < Li+
- c)K+ < Na+ < Rb+ < Li+
- d)Na+ < Li+ < K+ < Rb+
Correct answer is option 'B'. Can you explain this answer?
The ease of adsorption of the hydrated alkali metal ions on an ion-exchange resins follows the order :[2012]
a)
Li+ < K+ < Na+ < Rb+
b)
Rb+ < K+ <Na+ < Li+
c)
K+ < Na+ < Rb+ < Li+
d)
Na+ < Li+ < K+ < Rb+

|
Dipanjan Mehta answered |
All a lkali metal salts are ionic (except Lithium) and soluble in water due to the fact that cations get hydrated by water molecules. The degree of hydration depends upon the size of the cation. Smaller the size of a cation, greater is its hydration energy.
Relative ionic radii :
Relative ionic radii :
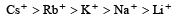
Relative ionic radii in water or relative degree of hydration:
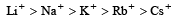
The compound A on heating gives a colourless gas and a residue that is dissolved in water to obtain B. Excess of CO2 is bubbled through aqueous solution of B, C is formed which is recovered in the solid form. Solid C on gentle heating gives back A. The compound is [2010]- a)CaSO4.2H2O
- b)CaCO3
- c)Na2CO3
- d)K2CO3
Correct answer is option 'B'. Can you explain this answer?
The compound A on heating gives a colourless gas and a residue that is dissolved in water to obtain B. Excess of CO2 is bubbled through aqueous solution of B, C is formed which is recovered in the solid form. Solid C on gentle heating gives back A. The compound is [2010]
a)
CaSO4.2H2O
b)
CaCO3
c)
Na2CO3
d)
K2CO3

|
Rajeev Sharma answered |
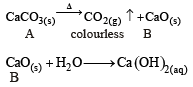
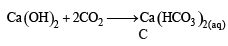
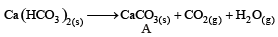
∴ Correct choice : (b)
The correct order of increasing thermal stability of K2CO3, MgCO3, CaCO3 and BeCO3 is[2007]- a)BeCO3< MgCO3 < CaCO3 < K2CO3
- b)MgCO3 < BeCO3 < CaCO3 < K2CO3
- c)K2CO3 < MgCO3 < CaCO3 < BeCO3
- d)BeCO3 < MgCO3 < K2CO3 < CaCO3
Correct answer is option 'A'. Can you explain this answer?
The correct order of increasing thermal stability of K2CO3, MgCO3, CaCO3 and BeCO3 is[2007]
a)
BeCO3< MgCO3 < CaCO3 < K2CO3
b)
MgCO3 < BeCO3 < CaCO3 < K2CO3
c)
K2CO3 < MgCO3 < CaCO3 < BeCO3
d)
BeCO3 < MgCO3 < K2CO3 < CaCO3

|
Ashwini Khanna answered |
As the basicity of metal hydroxides increases down the group from Be to Ba, the thermal stability of their carbonates also increases in the same order. Further group 1 compounds are more thermally stable than group 2 because their hydroxide are much basic than group 2 hydroxides therefore, the order of thermal stability is BeCO3 < MgCO3< CaCO3< K2CO3.
Calcium is obtained by the [1997]- a)electrolysis of solution of calcium chloride in water
- b)electrolysis of molten anhydrous calcium chloride or fused calcium chloride
- c)roasting of limestone
- d)reduction of calcium chloride with carbon
Correct answer is option 'B'. Can you explain this answer?
Calcium is obtained by the [1997]
a)
electrolysis of solution of calcium chloride in water
b)
electrolysis of molten anhydrous calcium chloride or fused calcium chloride
c)
roasting of limestone
d)
reduction of calcium chloride with carbon

|
Raghav Khanna answered |
Calcium is obtained by electrolysis of a fused mass consisting six parts calcium chloride and one part calcium fluoride at about 700°C in an electrolytic cell.
Which of the following oxides is not expected to react with sodium hydroxide? [2009]- a)CaO
- b)SiO2
- c)BeO
- d)B2O3
Correct answer is option 'A'. Can you explain this answer?
Which of the following oxides is not expected to react with sodium hydroxide? [2009]
a)
CaO
b)
SiO2
c)
BeO
d)
B2O3

|
Maya Sengupta answered |
NaOH is a strong alkali. It combines with acidic and amphoteric oxides to form salts.
Since CaO is a basic oxide hence does not reacts with NaOH.
Since CaO is a basic oxide hence does not reacts with NaOH.
Which of the following will react most vigorously with water- a)Li
- b)Rb
- c)K
- d)Na
Correct answer is option 'B'. Can you explain this answer?
Which of the following will react most vigorously with water
a)
Li
b)
Rb
c)
K
d)
Na
|
|
Ankita Menon answered |
Down the group , reactivity of s block metals increase.
Sodium is made by the electrolysis of a molten mixture of about 40% NaCl and 60% CaCl2 because [1995]- a)Ca++ can reduce NaCl to Na
- b)Ca++ can displace Na from NaCl
- c)CaCl2 helps in conduction of electricity
- d)this mixture has a lower melting point than NaCl
Correct answer is option 'D'. Can you explain this answer?
Sodium is made by the electrolysis of a molten mixture of about 40% NaCl and 60% CaCl2 because [1995]
a)
Ca++ can reduce NaCl to Na
b)
Ca++ can displace Na from NaCl
c)
CaCl2 helps in conduction of electricity
d)
this mixture has a lower melting point than NaCl

|
Maheshwar Saini answered |
Sodium is obtained by electrolytic reduction of its chloride. Melting point of chloride of sodium is high (803°C) so in order to lower its melting point(600°C), calcium chloride is added to it.
The alkali metals form salt-like hydrides by the direct synthesis at elevated temperature. The thermal stability of these hydrides decreases in which of the following orders ? [2008]- a)CsH > RbH > KH > NaH > LiH
- b)KH > NaH > LiH > CsH > RbH
- c)NaH > LiH > KH > RbH > CsH
- d)LiH > NaH > KH > RbH > CsH
Correct answer is option 'D'. Can you explain this answer?
The alkali metals form salt-like hydrides by the direct synthesis at elevated temperature. The thermal stability of these hydrides decreases in which of the following orders ? [2008]
a)
CsH > RbH > KH > NaH > LiH
b)
KH > NaH > LiH > CsH > RbH
c)
NaH > LiH > KH > RbH > CsH
d)
LiH > NaH > KH > RbH > CsH

|
Subhankar Datta answered |
The stability of alkali metal hydrides decreases from Li to Cs. It is due to the fact that M–H bonds becomes weaker with increase in size of alkali metals as we move down the group from Li to Cs. Thus the order of stability of hydrides is LiH > NaH > KH > RbH > CsH i.e. option (d) is correct answer.
Which one is the correct statement with reference to solubility of MgSO4 in water? [1996]- a)SO4 2– ion main ly contributes towards hydration energy
- b)Sizes of Mg2+ and SO42– are similar
- c)Hydration energy of MgSO4 is higher in comparison to its lattice energy
- d)Ionic potential (charge/radius ratio) of Mg2+ is very low
Correct answer is option 'C'. Can you explain this answer?
Which one is the correct statement with reference to solubility of MgSO4 in water? [1996]
a)
SO4 2– ion main ly contributes towards hydration energy
b)
Sizes of Mg2+ and SO42– are similar
c)
Hydration energy of MgSO4 is higher in comparison to its lattice energy
d)
Ionic potential (charge/radius ratio) of Mg2+ is very low

|
Maheshwar Saini answered |
MgSO4 is the only alkaline ear th metal sulphate which is soluble in water and for solubility hydration energy should be greater than lattice energy i.e hydration energy > lattice energy
Which one of the alkali metals, forms only, the normal oxide, M2O on heating in air ? [2012]- a)Rb
- b)K
- c)Li
- d)Na
Correct answer is option 'C'. Can you explain this answer?
Which one of the alkali metals, forms only, the normal oxide, M2O on heating in air ? [2012]
a)
Rb
b)
K
c)
Li
d)
Na

|
Raghav Khanna answered |
All the alkali metals when heated with oxygen form different types of oxides for example lithium forms lithium oxide (Li2O), sodium forms sodium peroxide (Na2O2), while K, Rb and Cs form their respective superoxides.
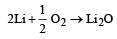
Chapter doubts & questions for The s - Block Elements - Chemistry 31 Years NEET Chapterwise Solved Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of The s - Block Elements - Chemistry 31 Years NEET Chapterwise Solved Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily