All Exams >
NEET >
Chemistry 31 Years NEET Chapterwise Solved Papers >
All Questions
All questions of Alcohols, Phenols and Ethers for NEET Exam
Which of the following will not form a yellow precipitate on heating with an alkaline solution of iodine? [2004]- a) CH3CH(OH)CH3
- b)CH3CH2CH(OH)CH3
- c)CH3OH
- d)CH3CH2OH
Correct answer is option 'C'. Can you explain this answer?
Which of the following will not form a yellow precipitate on heating with an alkaline solution of iodine? [2004]
a)
CH3CH(OH)CH3
b)
CH3CH2CH(OH)CH3
c)
CH3OH
d)
CH3CH2OH

|
Prasenjit Pillai answered |
CH3OH does not have -CH(OH)CH3 grouping hence it will not form yellow precipitate with an alkaline solution of iodine (haloform reaction).
How many isomers of C5H11 OH will be primary alcohols ? [1992]- a)5
- b)4
- c)2
- d)3.
Correct answer is option 'B'. Can you explain this answer?
How many isomers of C5H11 OH will be primary alcohols ? [1992]
a)
5
b)
4
c)
2
d)
3.

|
Gowri Nair answered |
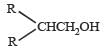
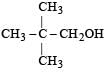
Four primary alcohols of C5H11OH are possible. These are:
(i) CH3CH2CH2CH2CH2OH
(ii)
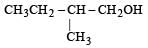
(iii)
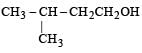
(iv)
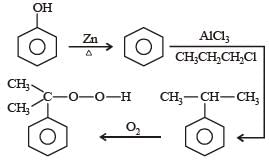
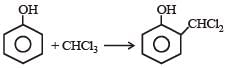
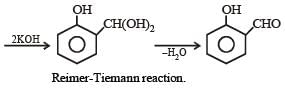
Phenol is distilled with Zn dust followed by Friedel Crafts alkylation with propyl chloride in the presence of AlCl3 to give a compound (B). (B) is oxidised in the presence of air to form the compound (C). The structural formula of (C) is [NEET Kar. 2013]- a)
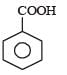
- b)
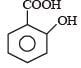
- c)
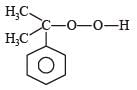
- d)
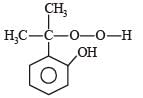
Correct answer is option 'C'. Can you explain this answer?
Phenol is distilled with Zn dust followed by Friedel Crafts alkylation with propyl chloride in the presence of AlCl3 to give a compound (B). (B) is oxidised in the presence of air to form the compound (C). The structural formula of (C) is [NEET Kar. 2013]
a)

b)

c)

d)


|
Ashwini Khanna answered |
Which of the following compounds will form hydrocarbon on reaction with Grignard reagent ?- a)CH2CHO
- b)CH3COCH3
- c) CH3CH2OH
- d)CH3CO2CH3
Correct answer is option 'C'. Can you explain this answer?
Which of the following compounds will form hydrocarbon on reaction with Grignard reagent ?
a)
CH2CHO
b)
CH3COCH3
c)
CH3CH2OH
d)
CH3CO2CH3
|
|
Chirag Joshi answered |
Formation of Hydrocarbon from Grignard Reagent
Grignard reagent is a very strong nucleophile and a powerful reducing agent. It reacts with various types of carbonyl compounds to form alcohols, which can further react to form hydrocarbons. However, not all carbonyl compounds can form hydrocarbons on reaction with Grignard reagent. Let's discuss the given options:
a) CH2CHO: This is formaldehyde, which has a carbonyl group. It can react with Grignard reagent to form a primary alcohol, but it cannot form a hydrocarbon.
b) CH3COCH3: This is acetone, which also has a carbonyl group. It can react with Grignard reagent to form a secondary alcohol, but it cannot form a hydrocarbon.
c) CH3CH2OH: This is ethanol, which has a hydroxyl group. It can react with Grignard reagent to form a secondary alcohol, which can further react to form an alkene (hydrocarbon).
d) CH3CO2CH3: This is ethyl acetate, which has an ester group. It can react with Grignard reagent to form a tertiary alcohol, but it cannot form a hydrocarbon.
Therefore, the correct answer is option 'C' (CH3CH2OH).
Grignard reagent is a very strong nucleophile and a powerful reducing agent. It reacts with various types of carbonyl compounds to form alcohols, which can further react to form hydrocarbons. However, not all carbonyl compounds can form hydrocarbons on reaction with Grignard reagent. Let's discuss the given options:
a) CH2CHO: This is formaldehyde, which has a carbonyl group. It can react with Grignard reagent to form a primary alcohol, but it cannot form a hydrocarbon.
b) CH3COCH3: This is acetone, which also has a carbonyl group. It can react with Grignard reagent to form a secondary alcohol, but it cannot form a hydrocarbon.
c) CH3CH2OH: This is ethanol, which has a hydroxyl group. It can react with Grignard reagent to form a secondary alcohol, which can further react to form an alkene (hydrocarbon).
d) CH3CO2CH3: This is ethyl acetate, which has an ester group. It can react with Grignard reagent to form a tertiary alcohol, but it cannot form a hydrocarbon.
Therefore, the correct answer is option 'C' (CH3CH2OH).
Which one of the following compounds will be most readily attacked by an electrophile ?[1989]- a)Chlorobenzene
- b)Benzene
- c)Phenol
- d)Toluene
Correct answer is option 'C'. Can you explain this answer?
Which one of the following compounds will be most readily attacked by an electrophile ?[1989]
a)
Chlorobenzene
b)
Benzene
c)
Phenol
d)
Toluene

|
Abhijeet Goyal answered |
Due to strong electron-donating effect of the OH group, the electron density in phenol is much higher than that in toluene, benzene and chlorobenzene and hence phenol is readily attacked by the electrophile.
When glycerol is treated with excess of HI, it produces: [2010]- a)glycerol triiodide
- b)2–iodopropane
- c)allyl iodide
- d)pr open e
Correct answer is option 'B'. Can you explain this answer?
When glycerol is treated with excess of HI, it produces: [2010]
a)
glycerol triiodide
b)
2–iodopropane
c)
allyl iodide
d)
pr open e

|
Nilanjan Chakraborty answered |
Glycerol when treated with excess HI produces 2–iodopropane
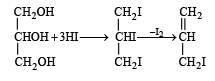
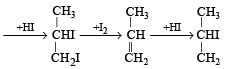
∴ Correct choice : (b)
When phenol is treated with excess bromine water. It gives [1992]- a)m-Bromophenol
- b)o-and p-Bromophenols
- c)2,4-Dibromophenol
- d)2,4, 6-Tribromophenol.
Correct answer is option 'D'. Can you explain this answer?
When phenol is treated with excess bromine water. It gives [1992]
a)
m-Bromophenol
b)
o-and p-Bromophenols
c)
2,4-Dibromophenol
d)
2,4, 6-Tribromophenol.
|
|
Soumya Nair answered |
With Br2 water, phenol gives 2, 4, 6-tribromophenol.
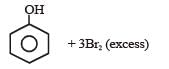
What is formed when a primary alcohol undergoes catalytic dehydrogenation ? [1993]- a)Aldeh yde
- b)Ketone
- c)Alkene
- d)Acid
Correct answer is option 'A'. Can you explain this answer?
What is formed when a primary alcohol undergoes catalytic dehydrogenation ? [1993]
a)
Aldeh yde
b)
Ketone
c)
Alkene
d)
Acid

|
sankalp raj answered |
In hydrogenation the primary alcohol convert into aldehyde but how dehydrogenation? cause this...
The stablest among the following is [1994]- a)CH3CH(OH)2
- b)ClCH2CH(OH)2
- c)(CH3)2 C (OH)2
- d)CCl3 CH (OH)2.
Correct answer is option 'D'. Can you explain this answer?
The stablest among the following is [1994]
a)
CH3CH(OH)2
b)
ClCH2CH(OH)2
c)
(CH3)2 C (OH)2
d)
CCl3 CH (OH)2.

|
Soumya Ahuja answered |
Due to –I-effect of the three C–Cl-bonding between CI and C-atom of the OH group, CCl3 CH (OH)2 is most stable.
Which of the following compounds can be used as antifreeze in automobile radiators ? [2012 M]- a)Methyl alcohol
- b)Glycol
- c)Nitrophenol
- d)Ethyl alcohol
Correct answer is option 'B'. Can you explain this answer?
Which of the following compounds can be used as antifreeze in automobile radiators ? [2012 M]
a)
Methyl alcohol
b)
Glycol
c)
Nitrophenol
d)
Ethyl alcohol

|
Ishaan Menon answered |
Glycol is used as an antifreeze in automobiles.
Which of the following is correct ? [2001]- a)On reduction of any aldehyde, secondary alcohol is formed
- b)Reaction of vegetable oil with H2SO4 gives glycerine
- c)Sucrose on reaction with NaCl gives invert sugar
- d)Alcoholic iodine gives iodoform with NaOH
Correct answer is option 'D'. Can you explain this answer?
Which of the following is correct ? [2001]
a)
On reduction of any aldehyde, secondary alcohol is formed
b)
Reaction of vegetable oil with H2SO4 gives glycerine
c)
Sucrose on reaction with NaCl gives invert sugar
d)
Alcoholic iodine gives iodoform with NaOH

|
Pankaj Kulkarni answered |
The compound which reacts fastest with Lucas reagent at room temperature is [1989]- a)Butan-1-ol
- b)Butan-2-ol
- c)2-Methyl propan-1-ol
- d)2-Methylpropan-2-ol
Correct answer is option 'D'. Can you explain this answer?
The compound which reacts fastest with Lucas reagent at room temperature is [1989]
a)
Butan-1-ol
b)
Butan-2-ol
c)
2-Methyl propan-1-ol
d)
2-Methylpropan-2-ol

|
Maya Sengupta answered |
The rates of reaction with lucas reagen t follows the order. 3° alcohol > 2° alcohol > 1° alcohol since carbocations are formed as intermediate, more stable the carbocation, higher will be the reactivity of the parent compound (alcohol). 2-Methylpropan-2-ol generates a 3º carbocation, so it will react fastest; other three generates either 1º or 2º carbocations.
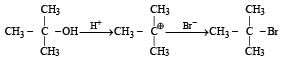
2-Methyl-2-propanol
HBr reacts fastest with - a)2-Mehtylpropan-1-ol
- b)2-Methylpropan-2-ol
- c)propan-2-ol
- d)propan-1-ol.
Correct answer is option 'B'. Can you explain this answer?
HBr reacts fastest with
a)
2-Mehtylpropan-1-ol
b)
2-Methylpropan-2-ol
c)
propan-2-ol
d)
propan-1-ol.
|
|
Roshni Desai answered |
Greater the stability of the intermediate carbocation, more reactive is the alcohol.
Since 2-methylpropan-2-ol generates 3° carbocation, therefore, it reacts fastest with HBr.
Since 2-methylpropan-2-ol generates 3° carbocation, therefore, it reacts fastest with HBr.
Increasing order of acid strength among p-methoxyphenol, p-methylphenol and p-nitrophenol is [1993]- a)p-Nitrophenol, p-Methoxyphenol, p-Methylphenol
- b)p-Methylphenol, p-Methoxyphenol, p-Nitrophenol
- c)p-Nitrophenol, p-Methylphenol, p-Methoxyphenol.
- d)p-Methoxyphenol, p-Methylphenol, p-Nitrophenol
Correct answer is option 'D'. Can you explain this answer?
Increasing order of acid strength among p-methoxyphenol, p-methylphenol and p-nitrophenol is [1993]
a)
p-Nitrophenol, p-Methoxyphenol, p-Methylphenol
b)
p-Methylphenol, p-Methoxyphenol, p-Nitrophenol
c)
p-Nitrophenol, p-Methylphenol, p-Methoxyphenol.
d)
p-Methoxyphenol, p-Methylphenol, p-Nitrophenol

|
Vaibhav Basu answered |
Electron-donating groups (– OCH3, – CH3 etc.) tend to decrease and electron withdrawing groups (– NO2, – OCH3 etc.) tend to increase the acidic character of phenols. Since – OCH3 is a more powerful electron-donating group than – CH3 group, therefore, p-methylphenol is slightly more acidic than p-methoxyphenol while pnitrophenol is the strongest acid. Thus, option (d), i.e. p-methoxyphenol, pmethylphenol, p-nitrophenol is correct.
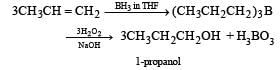
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]- a)KMnO4 (alkaline)
- b)Osmium tetraoxide (OsO4/CH2Cl2)
- c)B6H6 and alk. H2O2
- d)O3/Zn
Correct answer is option 'C'. Can you explain this answer?
Propene, CH3CH = CH2 can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion ? [1989]
a)
KMnO4 (alkaline)
b)
Osmium tetraoxide (OsO4/CH2Cl2)
c)
B6H6 and alk. H2O2
d)
O3/Zn

|
Soumya Ahuja answered |
KMnO4 (alkaline) and OsO4/CH2Cl2 are used for hydroxylation of double bond while O3/Zn is used for ozonolysis. Therefore, the right option is (c), i.e.,
1-Phenylethanol can be prepared by the reaction of benzaldehyde with [1997]- a)ethyl iodide and magnesium
- b)methyl iodide and magnesium
- c)methyl bromide and aluminium bromide
- d)methyl bromide
Correct answer is option 'B'. Can you explain this answer?
1-Phenylethanol can be prepared by the reaction of benzaldehyde with [1997]
a)
ethyl iodide and magnesium
b)
methyl iodide and magnesium
c)
methyl bromide and aluminium bromide
d)
methyl bromide
|
|
Dev Kumar answered |
CH3I + Mg----> CH3MgI (organometallic synthesis)
now carbonyl group on benzaldehyde undergoes mesomeric effect and develops positive charge on carbonyl carbon and negative charge on oxygen atom. thereafter CH3- attacks on carbonyl carbon and MgI+ attacks on carbonyl oxygen. hydrolysis is followed and 1-Phenylethanol is formed.
Ethylene oxide whent reated with Grignard reagent yields [2006]- a)tertiary alcohol
- b)cyclopropyl alcohol
- c)primary alcohol
- d)secondary alcohol
Correct answer is option 'C'. Can you explain this answer?
Ethylene oxide whent reated with Grignard reagent yields [2006]
a)
tertiary alcohol
b)
cyclopropyl alcohol
c)
primary alcohol
d)
secondary alcohol

|
Pankaj Banerjee answered |
Ethylene oxide when treated with Grignard Reagent gives primary alcohol.
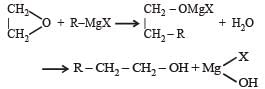
Methanol is industrially prepared by [1992]- a)Oxidation of CH4 by steam at 900°C
- b)Reduction of HCHO using LiAIH4
- c)Reaction HCHO with a solution of NaOH
- d)Reduction of CO using H2 and ZnO – Cr2O3.
Correct answer is option 'D'. Can you explain this answer?
Methanol is industrially prepared by [1992]
a)
Oxidation of CH4 by steam at 900°C
b)
Reduction of HCHO using LiAIH4
c)
Reaction HCHO with a solution of NaOH
d)
Reduction of CO using H2 and ZnO – Cr2O3.

|
Parth Iyer answered |
°C in the presence of a catalyst
b)Oxidation of CH4 by oxygen at 1200°C in the presence of a catalyst
c)Catalytic hydrogenation of CO2
d)Catalytic dehydration of ethanol
b)Oxidation of CH4 by oxygen at 1200°C in the presence of a catalyst
c)Catalytic hydrogenation of CO2
d)Catalytic dehydration of ethanol
Among the following four compounds[2010] (i) phenol (ii) methylphenol (iii) meta-nitrophenol (iv) para-nitrophenol the acidity order is :- a)ii > i > iii > iv
- b)iv > iii > i > ii
- c)iii > iv > i > ii
- d)i > iv > iii > ii
Correct answer is option 'B'. Can you explain this answer?
Among the following four compounds[2010] (i) phenol (ii) methylphenol (iii) meta-nitrophenol (iv) para-nitrophenol the acidity order is :
a)
ii > i > iii > iv
b)
iv > iii > i > ii
c)
iii > iv > i > ii
d)
i > iv > iii > ii

|
Arpita Tiwari answered |
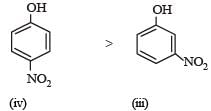
(– I and – M effects, (only – I effect) both increase acidity)
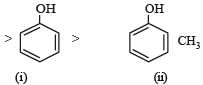
(+ I effect of CH3 group decreases acidity)
∴ Correct choice : (b)
∴ Correct choice : (b)
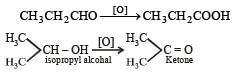
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?- a)PCl5 [2002]
- b)Reduction
- c)Oxidation with potassium dichromate
- d)Ozonolysis
Correct answer is option 'C'. Can you explain this answer?
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
a)
PCl5 [2002]
b)
Reduction
c)
Oxidation with potassium dichromate
d)
Ozonolysis

|
Snehal Shah answered |
Primary alcohol on oxidation give aldehyde which on further oxidation give carboxylic acid whereas secondary alcohols give ketone.
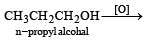
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI? [NEET 2013]- a)
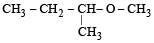
- b)
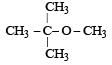
- c)
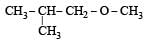
- d)
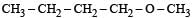
Correct answer is option 'B'. Can you explain this answer?
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI? [NEET 2013]
a)
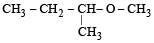
b)
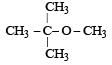
c)
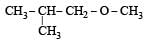
d)
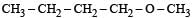

|
Shounak Nair answered |
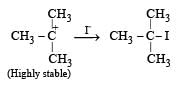
The reaction will proceed via SN1 or SN2 based on nature of alkyl group. If alkyl group attached is 3°. The reaction will proceed through the SN1 mechanism and if alkyl group is primary reaction will proceed through SN2 mechanism.
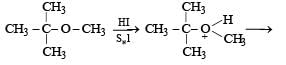
Which one of the following compounds has the most acidic nature? [2010]- a)
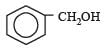
- b)
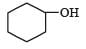
- c)
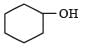
- d)
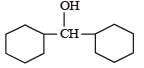
Correct answer is option 'B'. Can you explain this answer?
Which one of the following compounds has the most acidic nature? [2010]
a)

b)

c)

d)


|
Ritika Khanna answered |
Phenolis most acidic because its conjugate base is stabilised due to resonance, while the rest three compounds are alcohols, hence, their corrosponding conjugate bases do not exhibit resonance
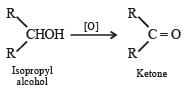
Which one of the following on oxidation gives a ketone ? [1993]- a)Primary alcohol
- b)secondary alcohol
- c)tertiary alcohol
- d)All of these
Correct answer is option 'B'. Can you explain this answer?
Which one of the following on oxidation gives a ketone ? [1993]
a)
Primary alcohol
b)
secondary alcohol
c)
tertiary alcohol
d)
All of these

|
Pankaj Banerjee answered |
Secon dary alcoh ols on oxidation give ketones.
Note : – Primary alcohols from aldehydes.
Note : – Primary alcohols from aldehydes.
H2COH · CH2OH on heating with periodic acid gives: [2009]- a)2 HCOOH
- b)
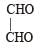
- c)
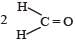
- d)2 CO2
Correct answer is option 'C'. Can you explain this answer?
H2COH · CH2OH on heating with periodic acid gives: [2009]
a)
2 HCOOH
b)

c)

d)
2 CO2

|
Naveen Menon answered |
1, 2 – Diols, when treated with an aqueous solution of periodic acid give aldehyde or ketones
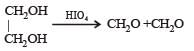
Note that a 1° alcohol gives CH2O. Since in glycol both the OH groups, are primary hence give 2 molecules of CH2O as by product.
The major organic product in the reaction, CH3 — O — CH(CH3)2 + HI → Product is [2006]- a)ICH2OCH(CH3)2
- b)
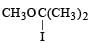
- c)CH3I + (CH3)2CHOH
- d)CH3OH + (CH3)2CHI
Correct answer is option 'C'. Can you explain this answer?
The major organic product in the reaction, CH3 — O — CH(CH3)2 + HI → Product is [2006]
a)
ICH2OCH(CH3)2
b)
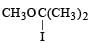
c)
CH3I + (CH3)2CHOH
d)
CH3OH + (CH3)2CHI

|
Lead Academy answered |
First HI will break into H+ and I-. Then H+ attacks on ether to form CH3O+HCH(CH3)2
Being an excellent nucleophile, I- attacks where it is easy to attack and so it will go for less bulky CH3 to form CH3I and (CH3)2CHOH.
Being an excellent nucleophile, I- attacks where it is easy to attack and so it will go for less bulky CH3 to form CH3I and (CH3)2CHOH.
The ionization constant of phenol is higher than that of ethanol because : [2000]- a)phenoxide ion is bulkier than ethoxide
- b)phenoxide ion is stronger base than ethoxide
- c)ph en oxide ion is st abilized t h r ough delocalization
- d)phenoxide ion is less stable than ethoxide
Correct answer is option 'C'. Can you explain this answer?
The ionization constant of phenol is higher than that of ethanol because : [2000]
a)
phenoxide ion is bulkier than ethoxide
b)
phenoxide ion is stronger base than ethoxide
c)
ph en oxide ion is st abilized t h r ough delocalization
d)
phenoxide ion is less stable than ethoxide

|
Naveen Menon answered |
The acidic nature of phenol is due to the formation of stable phenoxide ion in solution
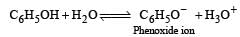
The phenoxide ion is stable due to resonance.
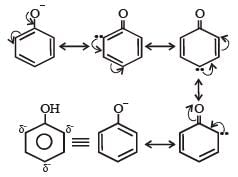
The negative charge is delocalized in the benzene ring which is a stabilizing factor in the phenoxide ion and increase acidity of phenol. wheras no resonance is possible in alkoxide ions (RO–)derived from alcohol. The negative charge is localized on oxygen atom.
Thus, alcohols are not acidic.
Thus, alcohols are not acidic.
Ethanol and dimethyl ether form a pair of functional isomers. The boiling point of ethanol is higher than that of dimethyl ether, due to the presence of [1993]- a)H-bonding in ethanol
- b)H-bonding in dimethyl ether
- c)CH3 group in ethanol
- d)CH3 group in dimethyl ether
Correct answer is option 'A'. Can you explain this answer?
Ethanol and dimethyl ether form a pair of functional isomers. The boiling point of ethanol is higher than that of dimethyl ether, due to the presence of [1993]
a)
H-bonding in ethanol
b)
H-bonding in dimethyl ether
c)
CH3 group in ethanol
d)
CH3 group in dimethyl ether

|
Subhankar Datta answered |
Due to H-bonding, the boiling point of ethanol is much higher than that of the isomeric diethyl ether.
Chapter doubts & questions for Alcohols, Phenols and Ethers - Chemistry 31 Years NEET Chapterwise Solved Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Alcohols, Phenols and Ethers - Chemistry 31 Years NEET Chapterwise Solved Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup

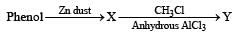

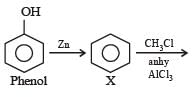
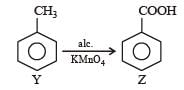

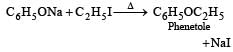
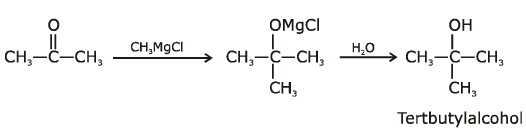
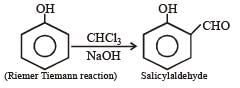
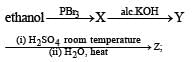
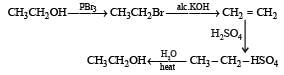

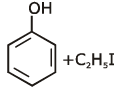
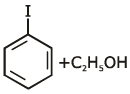
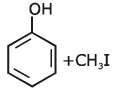
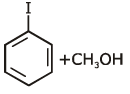
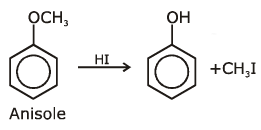
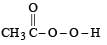
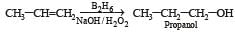
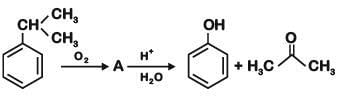
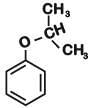
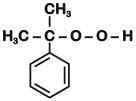
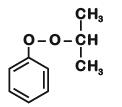
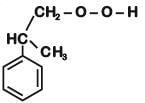
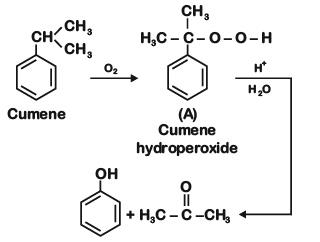
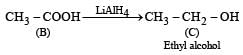
 the end product (C) is :[2012]
the end product (C) is :[2012]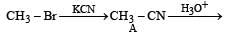
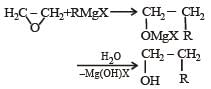
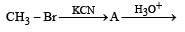
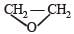 with RMgX leads toformation of [1998]
with RMgX leads toformation of [1998]