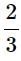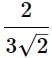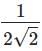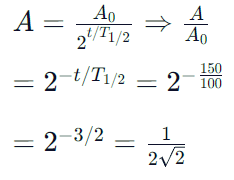All Exams >
NEET >
Chemistry 31 Years NEET Chapterwise Solved Papers >
All Questions
All questions of Nuclear Chemistry for NEET Exam
The half life of a substance in a certain enzymecatalysedreaction is 138s. The time required forthe concentration of the substance to fall from1.28 mg L–1 to 0.04 mg L–1, is : [2011]- a)414 s
- b)552 s
- c)690 s
- d)276 s
Correct answer is option 'C'. Can you explain this answer?
The half life of a substance in a certain enzymecatalysedreaction is 138s. The time required forthe concentration of the substance to fall from1.28 mg L–1 to 0.04 mg L–1, is : [2011]
a)
414 s
b)
552 s
c)
690 s
d)
276 s

|
Rajesh Datta answered |
For a first order reaction
Total time T = no. of half lives (n) × half life
(t1/2)
Total time T = no. of half lives (n) × half life
(t1/2)
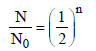
where n = no. of half lives
Give N0 (original amount) = 1.28 mg/ ℓ
N (amount of substance left after time T)
= 0.04 m/g l
Give N0 (original amount) = 1.28 mg/ ℓ
N (amount of substance left after time T)
= 0.04 m/g l
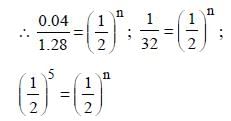
n = 5
T = 5 × 138
= 690
T = 5 × 138
= 690
The radioactive isotope 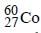 which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is- a)
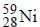
- b)
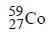
- c)
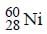
- d)
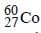
Correct answer is option 'C'. Can you explain this answer?
The radioactive isotope 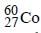 which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
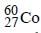 which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus isa)
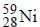
b)
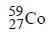
c)
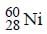
d)
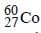

|
Kajal Bose answered |
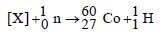
Balancing the mass and atomic numbers on
both sides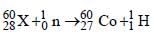
both sides
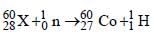
Thus X should be 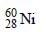
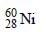
Number of neutrons in a parent nucleus X, which gives 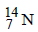 nucleus, after two successive β emissions, would be
nucleus, after two successive β emissions, would be- a)9
- b)6
- c)8
- d)7
Correct answer is option 'A'. Can you explain this answer?
Number of neutrons in a parent nucleus X, which gives 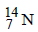 nucleus, after two successive β emissions, would be
nucleus, after two successive β emissions, would be
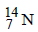 nucleus, after two successive β emissions, would be
nucleus, after two successive β emissions, would bea)
9
b)
6
c)
8
d)
7

|
Vaibhav Basu answered |
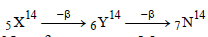
No. of neutrons = Mass number – No. of
proton =14 – 5 = 9
proton =14 – 5 = 9
One microgram of radioactive sodium 2411Na witha half-life of 15 hours was injected into a livingsystem for a bio-assay. How long will it take forthe radioactive subtance to fall up to 25% of theinitial value? [1996]- a)60 hours
- b)22.5 hours
- c)375 hours
- d)30 hours
Correct answer is option 'D'. Can you explain this answer?
One microgram of radioactive sodium 2411Na witha half-life of 15 hours was injected into a livingsystem for a bio-assay. How long will it take forthe radioactive subtance to fall up to 25% of theinitial value? [1996]
a)
60 hours
b)
22.5 hours
c)
375 hours
d)
30 hours

|
Mahi Shah answered |
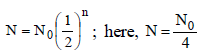
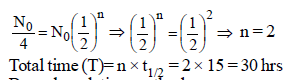
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]- a)5760 years
- b)11520 years
- c)17280 years
- d)23040 years
Correct answer is option 'C'. Can you explain this answer?
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]
a)
5760 years
b)
11520 years
c)
17280 years
d)
23040 years

|
Soumya Ahuja answered |
Half-life of 14C = 5760 yrs; Initial weight of
14C = 200 mg and final weight of 14C = 25 mg.
Quantity left after 5760 years = 200/2 = 100 mg
14C = 200 mg and final weight of 14C = 25 mg.
Quantity left after 5760 years = 200/2 = 100 mg
Similarly quantity left after another 5760
years (i.e 11520 years) = 100/2 = 50 mg
years (i.e 11520 years) = 100/2 = 50 mg
Quantity left after another 5760 years
(i.e. 17280 years) = 50/2 = 25 mg
(i.e. 17280 years) = 50/2 = 25 mg
Thus time taken by 200 mg of 14C to reduce
to 25 mg = (5760 + 5760 + 5760 ) years = 17280
years.
to 25 mg = (5760 + 5760 + 5760 ) years = 17280
years.
Alternative solution
As we know that
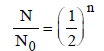
where N0 original amount of radioactive
sustacnce
N = Amount of substance remain after n half
lives
sustacnce
N = Amount of substance remain after n half
lives
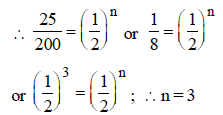
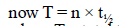
where T = total time
T = 3 × 5760 years = 17280 years
T = 3 × 5760 years = 17280 years
A human body required 0.01M activity ofradioactive substance after 24 hours. Half life ofradioactive substance is 6 hours. Then injectionof maximum activity of radioactive substance thatcan be injected will be [2001]- a)0.08 M
- b)0.04 M
- c)0.32 M
- d)0.16 M
Correct answer is option 'D'. Can you explain this answer?
A human body required 0.01M activity ofradioactive substance after 24 hours. Half life ofradioactive substance is 6 hours. Then injectionof maximum activity of radioactive substance thatcan be injected will be [2001]
a)
0.08 M
b)
0.04 M
c)
0.32 M
d)
0.16 M

|
Arindam Khanna answered |
Remaining activity = 0.01M
after 24 hrs
after 24 hrs
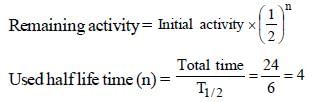

Initial activity = 0.01 × 16 = 0.16M
If an isotope of hydrogen has two neutrons in itsatom, its atomic number and atomic mass numberwill respectively be [1992]- a)2 and 1
- b)3 and 1
- c)1 and 1
- d)1 and 3.
Correct answer is option 'D'. Can you explain this answer?
If an isotope of hydrogen has two neutrons in itsatom, its atomic number and atomic mass numberwill respectively be [1992]
a)
2 and 1
b)
3 and 1
c)
1 and 1
d)
1 and 3.

|
Sneha Basak answered |
As number of neutron = Mass number
– atomic number
Give number of neutron = 2
∴ Mass number will be 3 and atomic number
will be one.
– atomic number
Give number of neutron = 2
∴ Mass number will be 3 and atomic number
will be one.
Carbon - 14 dating method is based on the factthat: [1997]- a)C-14 fraction is same in all objects
- b)C-14 is highly insoluble
- c)Ratio of carbon-14 and carbon-12 is constant
- d)all the above
Correct answer is option 'C'. Can you explain this answer?
Carbon - 14 dating method is based on the factthat: [1997]
a)
C-14 fraction is same in all objects
b)
C-14 is highly insoluble
c)
Ratio of carbon-14 and carbon-12 is constant
d)
all the above
|
|
Meera Singh answered |
Carbon dating is a technique used to determine the approximate age of once-living materials. It is based on the decay rate of the radioactive carbon isotope 14C, a form of carbon taken in by all living organisms while they are alive.
When a radioactive element emits successivelyone α-particle and two β-particles, the massnumber of the daughter element [1999]- a)is reduced by 4 units
- b)remains the same
- c)is reduced by 2 units
- d)is increased by 2 units
Correct answer is option 'A'. Can you explain this answer?
When a radioactive element emits successivelyone α-particle and two β-particles, the massnumber of the daughter element [1999]
a)
is reduced by 4 units
b)
remains the same
c)
is reduced by 2 units
d)
is increased by 2 units

|
Vaibhav Basu answered |
Mass number is effected by emmision of α
particle while β particle has negligible mass
does not effect mass number. e.g
particle while β particle has negligible mass
does not effect mass number. e.g
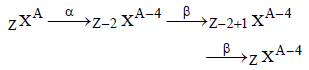
If species 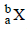 emits firstly a positron, then two α and two β and in last one α and finally converted to species
emits firstly a positron, then two α and two β and in last one α and finally converted to species 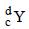 , so correct relation is
, so correct relation is- a)c = a – 5, d = b – 12
- b)c = a – 6, d = b – 8
- c)c = a – 4, d = b – 12
- d)c = a – 5, d = b – 8
Correct answer is option 'A'. Can you explain this answer?
If species 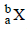 emits firstly a positron, then two α and two β and in last one α and finally converted to species
emits firstly a positron, then two α and two β and in last one α and finally converted to species 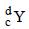 , so correct relation is
, so correct relation is
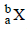 emits firstly a positron, then two α and two β and in last one α and finally converted to species
emits firstly a positron, then two α and two β and in last one α and finally converted to species 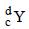 , so correct relation is
, so correct relation isa)
c = a – 5, d = b – 12
b)
c = a – 6, d = b – 8
c)
c = a – 4, d = b – 12
d)
c = a – 5, d = b – 8

|
Prashanth Dasgupta answered |
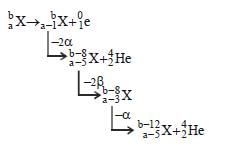
Emission of an alpha particle leads to a [1989]- a)Decrease of 2 units in the charge of the atom
- b)Increase of 2 units in the mass of the atom
- c)Decrease of 2 units in the mass of the atom
- d)Increase of 4 units in the mass of the atom.
Correct answer is option 'A'. Can you explain this answer?
Emission of an alpha particle leads to a
[1989]
a)
Decrease of 2 units in the charge of the atom
b)
Increase of 2 units in the mass of the atom
c)
Decrease of 2 units in the mass of the atom
d)
Increase of 4 units in the mass of the atom.

|
Diya Datta answered |
Emission of α -particle ) 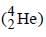 leads to
leads to
decrease of 2 units of charge. e.g
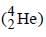 leads to
leads todecrease of 2 units of charge. e.g
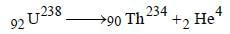
A nuclide of an alkaline earth metal undergoesradioactive decay by emission of the α-particles in succession. The group of theperiodic table to which the resulting daughterelement would belong is [2005]- a)Gr. 4
- b)Gr. 6
- c)Gr. 14
- d)Gr. 16
Correct answer is option 'C'. Can you explain this answer?
A nuclide of an alkaline earth metal undergoesradioactive decay by emission of the α-particles in succession. The group of theperiodic table to which the resulting daughterelement would belong is [2005]
a)
Gr. 4
b)
Gr. 6
c)
Gr. 14
d)
Gr. 16

|
Maheshwar Saini answered |
When IIA group element (Ra) emits one
α-particle its group no. decreases by two
unit. i.e., go into zero group (Gr. 16) But as
it is radioactive thus due to successive
emission last product is Pb i.e., (Gr.14).
α-particle its group no. decreases by two
unit. i.e., go into zero group (Gr. 16) But as
it is radioactive thus due to successive
emission last product is Pb i.e., (Gr.14).
In a radioactive decay, an emitted electron comesfrom [1994]- a)The nucleus of atom
- b)The orbit with principal quantum number 1
- c)the inner orbital of the atom
- d)the outermost orbit of the atom.
Correct answer is option 'A'. Can you explain this answer?
In a radioactive decay, an emitted electron comesfrom [1994]
a)
The nucleus of atom
b)
The orbit with principal quantum number 1
c)
the inner orbital of the atom
d)
the outermost orbit of the atom.

|
Sarthak Saini answered |
When a radioactive elements emits α or β
particle the new element formed may have
unstable nucleus. It may further disintegrate
by emitting α- or β particle forming a new
element. This process of integration may
continue till end product formed is a stable
compound.
particle the new element formed may have
unstable nucleus. It may further disintegrate
by emitting α- or β particle forming a new
element. This process of integration may
continue till end product formed is a stable
compound.
The radioactive isotope, tritium, 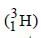 has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]
has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]- a)8 mg
- b)1 mg
- c)2 mg
- d)4 mg
Correct answer is option 'C'. Can you explain this answer?
The radioactive isotope, tritium, 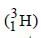 has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]
has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]
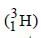 has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]
has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]a)
8 mg
b)
1 mg
c)
2 mg
d)
4 mg

|
Abhiram Nair answered |
Given t1/2 = 12.3 years
Initial amount (N0) = 32 mg
Total time = 49.2 years
Initial amount (N0) = 32 mg
Total time = 49.2 years
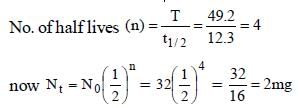
Hence 32 mg becomes 2 mg in 49.2 years
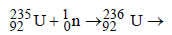 fission products + neutrons + 3.20 × 10–11 J The energy released when 1 g of finally undergoes fission is
fission products + neutrons + 3.20 × 10–11 J The energy released when 1 g of finally undergoes fission is- a)12.75 × 108 kJ
- b)16.40 × 107 kJ
- c)8.20 × 107 kJ
- d)6.50 × 106 kJ
Correct answer is option 'C'. Can you explain this answer?
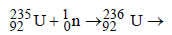
fission products + neutrons + 3.20 × 10–11 J The energy released when 1 g of finally undergoes fission is
a)
12.75 × 108 kJ
b)
16.40 × 107 kJ
c)
8.20 × 107 kJ
d)
6.50 × 106 kJ

|
Sneha Basak answered |
1 atom of 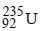 on fission gives energy
on fission gives energy
= 3.2 × 10–11 J
6.023 × 1023 atom (1 mole) on fission gives
energy = 3.2 × 10–11 × 6.023 × 1023 J
235 gm of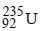 on fission gives energy
on fission gives energy
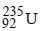 on fission gives energy
on fission gives energy= 3.2 × 10–11 J
6.023 × 1023 atom (1 mole) on fission gives
energy = 3.2 × 10–11 × 6.023 × 1023 J
235 gm of
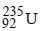 on fission gives energy
on fission gives energy 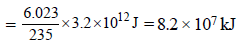
Chapter doubts & questions for Nuclear Chemistry - Chemistry 31 Years NEET Chapterwise Solved Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Nuclear Chemistry - Chemistry 31 Years NEET Chapterwise Solved Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup


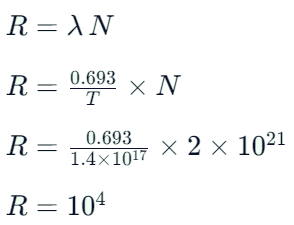
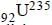 nucleus absorbs a neutron and disintegrates into
nucleus absorbs a neutron and disintegrates into 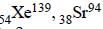 and x. So what will be the product x? [2002]
and x. So what will be the product x? [2002]