NEET Exam > NEET Questions > Half-life for radioactive 14C is 5760 years. ...
Start Learning for Free
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]
- a)5760 years
- b)11520 years
- c)17280 years
- d)23040 years
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg ...
Half-life of 14C = 5760 yrs; Initial weight of
14C = 200 mg and final weight of 14C = 25 mg.
Quantity left after 5760 years = 200/2 = 100 mg
14C = 200 mg and final weight of 14C = 25 mg.
Quantity left after 5760 years = 200/2 = 100 mg
Similarly quantity left after another 5760
years (i.e 11520 years) = 100/2 = 50 mg
years (i.e 11520 years) = 100/2 = 50 mg
Quantity left after another 5760 years
(i.e. 17280 years) = 50/2 = 25 mg
(i.e. 17280 years) = 50/2 = 25 mg
Thus time taken by 200 mg of 14C to reduce
to 25 mg = (5760 + 5760 + 5760 ) years = 17280
years.
to 25 mg = (5760 + 5760 + 5760 ) years = 17280
years.
Alternative solution
As we know that
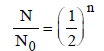
where N0 original amount of radioactive
sustacnce
N = Amount of substance remain after n half
lives
sustacnce
N = Amount of substance remain after n half
lives
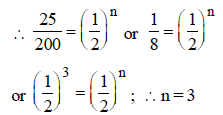
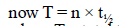
where T = total time
T = 3 × 5760 years = 17280 years
T = 3 × 5760 years = 17280 years
Most Upvoted Answer
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg ...
Understanding Half-Life
The half-life of a radioactive substance is the time required for half of the substance to decay. For Carbon-14 (14C), this period is 5760 years.
Calculating the Time for Reduction
To determine how long it takes for 200 mg of 14C to reduce to 25 mg, we first need to find out how many half-lives are involved in this reduction.
Initial and Final Amounts
- Initial amount (N0): 200 mg
- Final amount (N): 25 mg
Half-Life Formula
The relationship can be expressed as:
N = N0 * (1/2)^(t/T)
Where:
- t = total time
- T = half-life period (5760 years)
Finding the Number of Half-Lives
To find how many half-lives it takes to go from 200 mg to 25 mg, we can set up the equation:
200 mg * (1/2)^n = 25 mg
Solving for n:
- 200 mg = 2^n * 25 mg
- 8 = 2^n
- n = 3
This means it takes 3 half-lives to reduce from 200 mg to 25 mg.
Calculating Total Time
Now, we multiply the number of half-lives by the duration of one half-life:
t = n * T
t = 3 * 5760 years
t = 17280 years
Conclusion
Thus, it will take 17280 years for 200 mg of 14C to reduce to 25 mg, making the correct answer option C.
The half-life of a radioactive substance is the time required for half of the substance to decay. For Carbon-14 (14C), this period is 5760 years.
Calculating the Time for Reduction
To determine how long it takes for 200 mg of 14C to reduce to 25 mg, we first need to find out how many half-lives are involved in this reduction.
Initial and Final Amounts
- Initial amount (N0): 200 mg
- Final amount (N): 25 mg
Half-Life Formula
The relationship can be expressed as:
N = N0 * (1/2)^(t/T)
Where:
- t = total time
- T = half-life period (5760 years)
Finding the Number of Half-Lives
To find how many half-lives it takes to go from 200 mg to 25 mg, we can set up the equation:
200 mg * (1/2)^n = 25 mg
Solving for n:
- 200 mg = 2^n * 25 mg
- 8 = 2^n
- n = 3
This means it takes 3 half-lives to reduce from 200 mg to 25 mg.
Calculating Total Time
Now, we multiply the number of half-lives by the duration of one half-life:
t = n * T
t = 3 * 5760 years
t = 17280 years
Conclusion
Thus, it will take 17280 years for 200 mg of 14C to reduce to 25 mg, making the correct answer option C.

|
Explore Courses for NEET exam
|

|
Question Description
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer?.
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]a)5760 yearsb)11520 yearsc)17280 yearsd)23040 yearsCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















